Self-Administered Nasal Flu Shot Approval Under FDA Review
For the first time, an influenza vaccine sprayed into the nose that protects people against the seasonal flu has been accepted for review by the U.S. Food and Drug Administration (FDA).
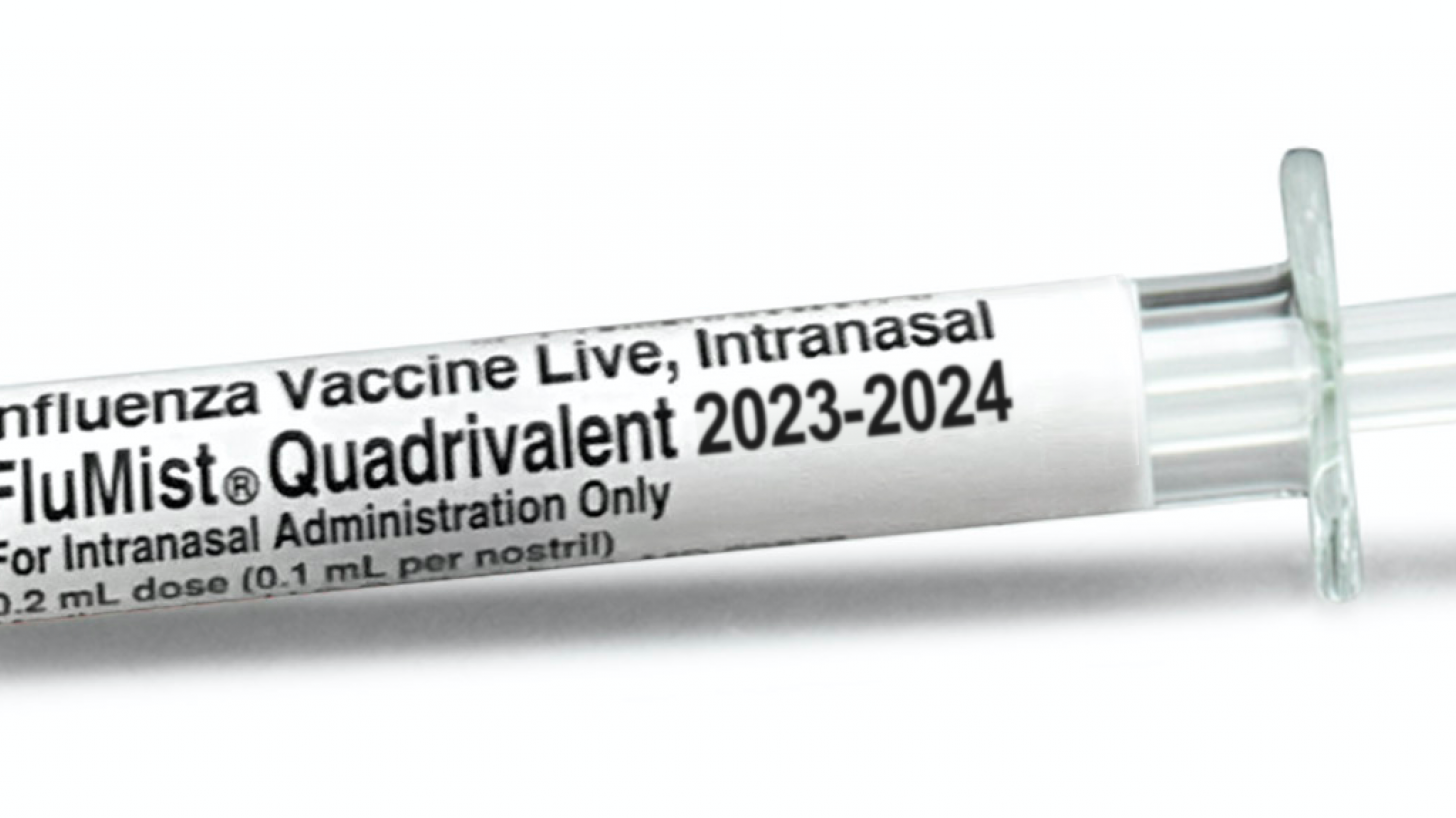
If approved by the FDA, FLUMIST® QUADRIVALENT will be the first live nasal influenza vaccine available to be self-administered by eligible patients or administered by caregivers.
AstraZeneca today announced its Supplemental Biologics License Application (sBLA) for the approval of a self- or caregiver-administered option for FLUMIST® QUADRIVALENT (Influenza Vaccine Live, Intranasal), a needle-free nasal spray,
The sBLA is supported by a usability study that confirmed that adults over 18 could self-administer or administer FLUMIST QUADRIVALENT to eligible patients 2-49 years of age when given instructions for use without any additional guidance.
The FDA's Prescription Drug User Fee Act date is expected during the first quarter of 2024. If approved, FLUMIST QUADRIVALENT is anticipated to be available for self-administration in the U.S. for the 2024/2025 flu season.
This means people could be able to get a flu vaccination while at their homes next year.
Nasal vaccines bypass Trypanophobia, an overlooked condition that affects virtually all medical procedures. Estimates show that as many as 2 in 3 children and 1 in 4 adults have intense fears around needles.
Ravi Jhaveri, MD, Division Head, Infectious Disease; Virginia H. Rogers Professor in Infectious Diseases, Professor of Pediatrics, Northwestern University School of Medicine, commented in a press release on October 24, 2023, ....." The ability for individuals and parents to choose where to administer an injection-free flu vaccine could help increase access and, subsequently, vaccination rates, and greatly benefit those most impacted by this serious and contagious respiratory illness."
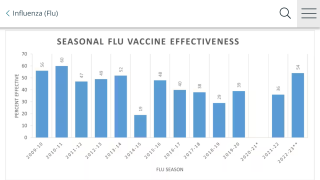
FLUMIST QUADRIVALENT is aleady an FDA-approved influenza vaccine. It is sprayed into the nose and has extensive data demonstrating comparable effectiveness and acceptable safety relative to other flu vaccines.
The most common side effects are runny or stuffy nose, sore throat, and fever over 100°F.
On September 5, 2023, AstraZeneca announced FLUMIST® QUADRIVALENT nasal vaccine doses were available in the U.S.
You should not get FLUMIST QUADRIVALENT if you have a severe allergy to eggs or any inactive ingredient in the vaccine, have ever had a life-threatening reaction to influenza vaccinations, or are 2 through 17 taking medicines containing aspirin. Children or adolescents should not be given aspirin for four weeks after getting FLUMIST QUADRIVALENT unless their healthcare provider tells them otherwise.
And inform your healthcare provider if you or your child are currently wheezing; have a history of wheezing if under five years old; have had Guillain-Barré syndrome; have a weakened immune system or live with someone who has a severely weakened immune system; have problems with your heart, kidneys, or lungs; have diabetes; are pregnant or nursing; or are taking a medication used to treat influenza.
Like most flu shots, FLUMIST QUADRIVALENT may not prevent influenza in everyone vaccinated. FLUMIST is currently available in the U.S. and the United Kingdom.
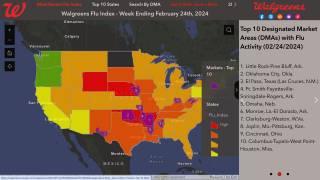
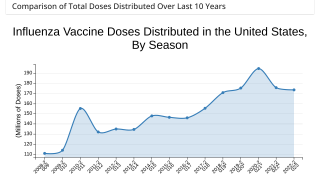
As of October 14, 2023, 136.94 million flu shots had been distributed in the U.S. for the 2023-2024 flu season.
Our Trust Standards: Medical Advisory Committee