Flu Shot Shoulder Injuries Can Be Prevented
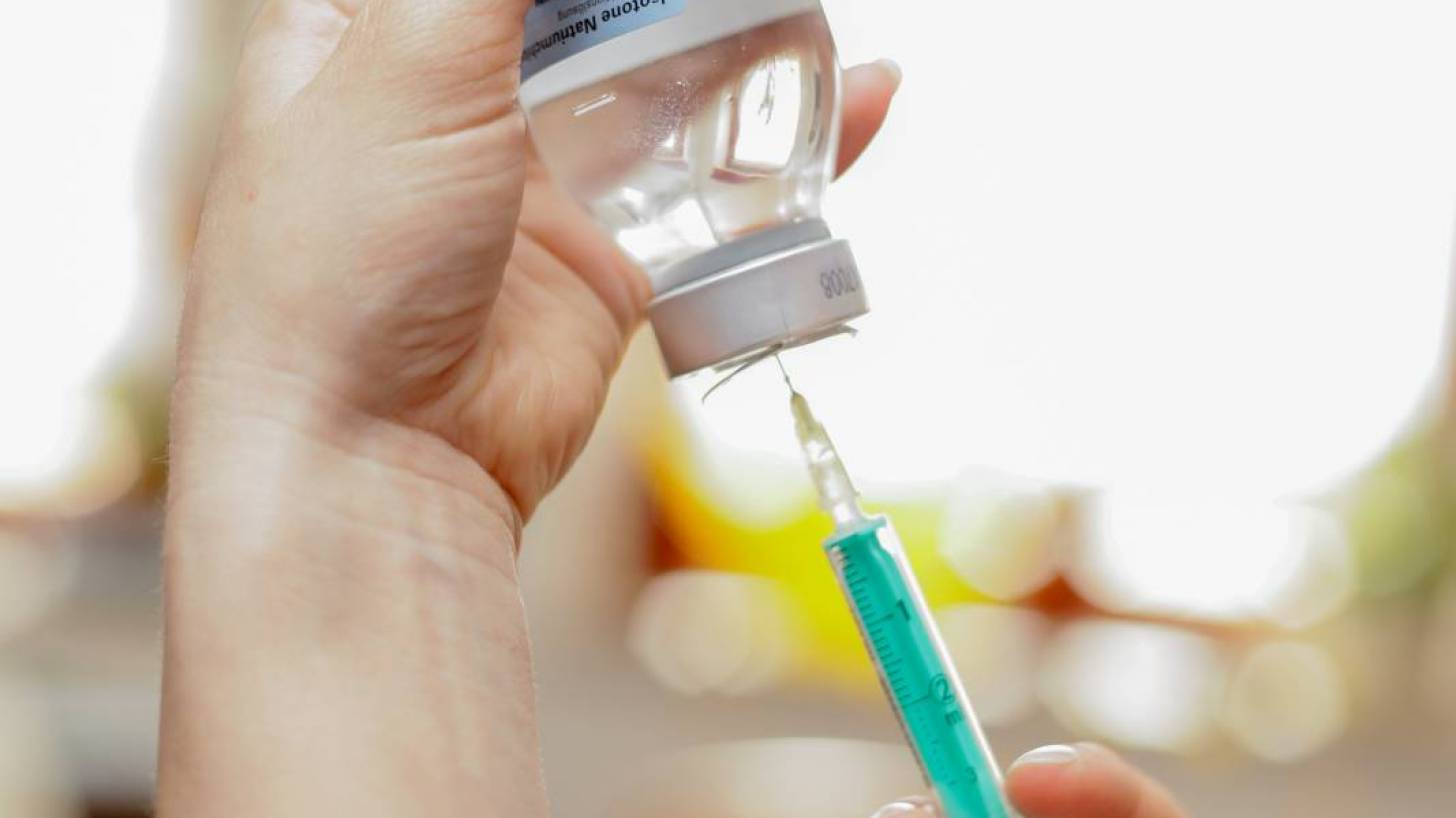
The University of Waterloo’s new study reiterates the need for healthcare professionals, including pharmacists, to take certain precautions to minimize the risk of vaccination patients suffering a Shoulder Injury Related to Vaccine Administration (SIRVA).
SIRVA’s are an understudied phenomenon that people may experience after receiving an improperly administered vaccination.
The injury occurs when a needle injection is administered too high in the arm, and the vaccine is delivered to the shoulder capsule, instead of the deltoid muscle.
It is common to experience a dull muscle ache after a vaccine injection, but that pain disappears within a few days.
By contrast, a SIRVA will result in pain that begins within 48 hours of vaccine administration and does not improve with over-the-counter painkiller medications.
"With flu season underway both the public and healthcare providers should understand how to recognize and respond to SIRVA," said Kelly Grindrod, a professor in the School of Pharmacy at Waterloo.
Dr. Grindord said in a press release there are strategies we can adopt to decrease the likelihood of experiencing SIRVA, such as:
- When going for your flu shot, wear a sleeveless shirt or a shirt where the sleeves can easily be rolled up,
- Don't pull the neck of your shirt down as this can lead to a vaccine being injected into the shoulder instead of the arm,
- Putting your hand on your hip with your elbow out and away from the body will also help relax the deltoid muscle where the injection is going.
"In patients who experience SIRVA, months may pass by, and patients will still complain of increasing pain, weakness, and impaired mobility in the injected arm. Simple actions like lifting your arm to brush your teeth can cause pain," said Dr. Grindrod.
"It's important that healthcare providers learn to recognize these signs of SIRVA so that we can access appropriate treatment," said Dr. Grindrod.
People experiencing these symptoms should talk to their doctor, nurse or pharmacist, as soon as possible, says the Centers for Disease Control and Prevention (CDC).
An ultrasound scan may be necessary to diagnose SIRVA and determine the level and type of damage. SIRVA treatment options include a corticosteroid injection to the shoulder or physiotherapy.
Dr. Grindrod and the study co-authors conducted a review of the literature to develop resources that teach healthcare providers about SIRVA and how to avoid it by using proper vaccination landmarking techniques.
When there is a legitimate shoulder injury from vaccinations in the USA, the National Vaccine Injury Compensation Program (VICP) may provide financial compensation to individuals who file a petition and are found to have been injured by a VICP-covered vaccine.
During 2015, the 21st Century Cures Act made several amendments to the National Childhood Vaccine Injury Act of 1986, which is the VICP authorizing legislation.
Within the 21st Century Cures Act, the Secretary of Health and Human Services published an important rule change in the vaccine court in favor of the injured and gives individuals an easier path to recovery.
Moreover, the 2015 rule change effectively reopens the statute of limitations period for cases that were previously time-barred. And, the statute of limitations in vaccine-injury cases is not state specific.
In addition, anyone who suffers a SIRVA injury within 48 hours of receipt of any vaccine on the Vaccine Injury Table, will also be given the presumption of causation.
“On behalf of attorneys that represent those injured from the administration of vaccines, we hope that this rule change will streamline the process for those injured to recover from the Vaccine Injury Compensation Fund,” said David J. Carney, an attorney at Anapol Weiss.
“Since the Vaccine Program exists, it is critical for administrators of vaccines, such as primary care physicians and pharmacies, to understand that these shoulder injuries can and do occur and that the administrators have an obligation to educate the injured about the Vaccine Injury Compensation Program for monetary recourse,” said Carney.
Any individual, of any age, who received a covered vaccine and believes he or she was injured, as a result, can file a petition.
Parents, legal guardians, and legal representatives can file on behalf of children, disabled adults, and individuals who are deceased.
The Vaccine Injury Compensation Program filing process is as follows:
- An individual files a petition with the U.S. Court of Federal Claims.
- The U.S. Department of Health and Human Services medical staff reviews the petition, determines if it meets the medical criteria for compensation and makes a preliminary recommendation.
- The U.S. Department of Justice develops a report that includes the medical recommendation and legal analysis and submits it to the Court.
- The report is presented to a court-appointed special master, who decides whether the petitioner should be compensated, often after holding a hearing in which both parties can present evidence. If compensation is awarded, the special master determines the amount and type of compensation.
- The Court orders the U.S. Department of Health and Human Services to award compensation. Even if the petition is dismissed, if certain requirements are met, the Court may order the Department to pay attorneys' fees and costs.
The special master's decision may be appealed and petitioners who reject the decision of the court (or withdraw their petitions within certain timelines) may file a claim in civil court against the vaccine company and/or the healthcare provider who administered the vaccine.
Our Trust Standards: Medical Advisory Committee
- How to help protect yourself from vaccine administration injury
- Getting it in the right spot: Shoulder injury related to vaccine administration (SIRVA) and other injection site events
- Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System
- National Vaccine Injury Compensation Program
- Program Description
- Shoulder Injuries Now Included in Vaccine Compensation Program
- National Vaccine Compensation Program Awards 485 Cases




























