Two Mosquito-Transmitted Viruses Causing Microcephaly in Newborns

According to the Pan American Health Organization (PAHO), scientists in Brazil believe that the Oropouche virus (OROV) may cause stillbirths and neurological defects in babies who are infected in the womb, similar to the Zika virus.
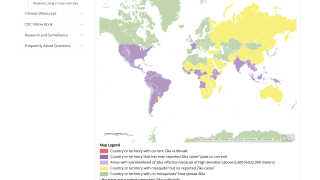
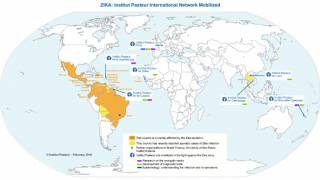
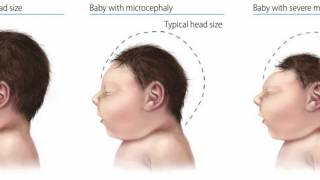
Since 2013, 31 countries and territories in the Americas have reported cases of microcephaly (head shape issues) and other central nervous system malformations associated with Zika infection.
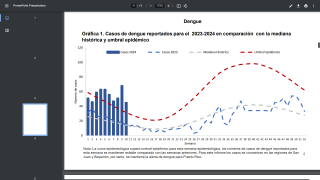
On July 12, 2024, the Brazilian Ministry of Health reported four cases of microcephaly in newborns from infected mothers and one potential fetal death associated with OROV.
In this communication, Brazil also reported that, in June 2024, a retrospective analysis of serum and cerebrospinal fluid samples collected and stored for arbovirus research, which had tested negative for dengue, chikungunya, Zika, and West Nile virus.
In this study, four newborns with microcephaly were detected with IgM class antibodies against OROV in serum samples and cerebrospinal fluid.
These researchers wrote that the studies' limitations do not allow for establishing a causal relationship between OROV infection and neurological malformations.
Brazil's health ministry now recommends health professionals closely monitor pregnant people infected with Oropouche, which was initially reported in Trinidad and Tobago in 1955.
In a PAHO Epidemiological Alert issued on July 17, 2024, the PAHO asked other countries to be on the lookout for similar cases. For example, in May 2024, Cuba's Ministry of Public Health reported 74 Oropouche cases.
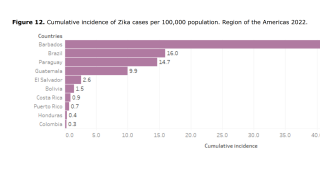
These OROV cases are similar to those during Brazil's previous and ongoing Zika outbreaks. As of July 2024, Brazil has reported over 24,000 Zika cases this year.
Additionally, Zika cases have been confirmed in Puerto Rico and Costa Rica in 2024.
From a prevention perspective, no U.S. FDA-approved vaccines targeting Zika and Oropouche viruses are available in 2024.
However, Zika vaccine candidates are conducting clinical research.
In March 2024, Valneva SE announced initiating a Phase 1 clinical trial to investigate the safety and immunogenicity of VLA1601, its second-generation adjuvanted inactivated vaccine candidate against Zika.
Our Trust Standards: Medical Advisory Committee