Ebola Preparedness Accelerates
The US government announced it is expanding the Strategic National Stockpile supply of vaccines and medications to better prepare for an Ebola outbreak.
Hundreds of thousands of Americans could be protected from or treated for Ebola infections through the purchase of vaccines and medications by the Department of Health and Human Services (HHS).
The vaccines and drugs are the first for Ebola to receive Project BioShield funding to prepare the USA for Ebola and other severe, highly infectious biothreats, either natural or man-made.
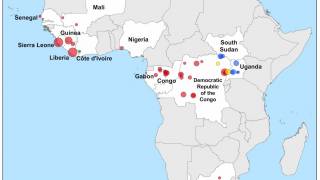
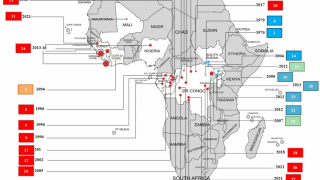
According to the World Health Organization (WHO), during the 2014-2016 outbreak, more than 28,600 cases of Ebola virus infection were suspected, probable or confirmed and more than 11,000 people died.
WHO officials believe multiple vaccine strategies will likely be needed to battle Ebola outbreaks.
“Today we are prepared to add four Ebola countermeasures to the stockpile whereas three years ago, very few products were even in early stages of development,” said BARDA Director Rick Bright, Ph.D. Biomedical Advanced Research and Development Authority (BARDA), is part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within HHS.
BARDA could purchase up to 1.13 million regimens of vaccine, including a single-dose vaccine from Merck and a two-dose vaccine from Janssen Vaccines.
In addition, BARDA will purchase a therapeutic drug from Mapp Biopharmaceutical, Inc., and a therapeutic drug from Regeneron Pharmaceuticals, Inc.
Merck’s single-shot vaccine approach aims to stop the spread of a virus by vaccinating everyone a patient came in contact with and everyone who came in contact with the patient’s contacts.
Janssen’s vaccine is a two-dose vaccine regimen that would be used to prevent illness in people who have not been exposed to Ebola but could be, such as health care workers and the general public.
Under the agreement with Mapp Biopharmaceutical, BARDA will purchase the therapeutic drug ZMapp™ under Project BioShield. ZMapp is a combination of three monoclonal antibodies that bind certain proteins in the virus cell to neutralize the virus, decreasing the amount of the virus in the body that the patient's immune system has to fight.
In addition, BARDA will initially provide $40.4 million for late-stage development and an initial purchase of REGN3470-3471-3479 from Regeneron Pharmaceuticals, Inc. This medication is a monoclonal antibody drug manufactured using specialized CHO mammalian (Chinese hamster ovary) cells.
For more information on public health and medical preparedness, and to learn more about partnering with BARDA in medical countermeasure development visit.
Our Trust Standards: Medical Advisory Committee
- HHS accelerates development of first Ebola vaccines and drugs
- Ebola virus disease
- Regeneron Announces New Collaborations with HHS to Develop Antibodies Against Ebola, Influenza and Multiple Other Emerging Patho
- MappBio Announces Contracts with BARDA for ZMappTM and Additional Filovirus Medical Countermeasures