Most Men Benefit from Initial and Catch-Up Cancer Prevention Vaccination

The Lancet Infectious Disease published the results from an extensive cancer prevention phase 3 study on November 12, 2021, supporting quadrivalent HPV vaccination in men, including catch-up vaccinations.
The Gardasil quadrivalent human papillomavirus (HPV) vaccine was shown to prevent infections and lesions related to HPV6, 11, 16, and 18 in men aged 16–26 years.
The researchers assessed the incidences of external genital warts related to HPV6 or 11 and external genital lesions and anal dysplasia associated with HPV6, 11, 16, or 18, over ten years of follow-up.
The 3-year Base Study was an international, double-blind, randomized, placebo-controlled trial done at 71 sites in 18 countries.
The Vaccination Period for the Base Study encompassed Day 1 through Month 7, during which time participants received qHPV vaccination at Day 1, Month 2, and Month 6. Follow-up for the Base Study encompassed Month 7 through Month 36.
And the 7-year, open-label, long-term follow-up extension study was done at 46 centers in 16 countries.
Between August 2010 and April 2017, 1,803 participants were enrolled in the long-term follow-up study, of whom 936 (827 heterosexual men and 109 MSM) were included in the early vaccination group and 867 (739 heterosexual men and 128 MSM) were included in the catch-up vaccination group.
In early vaccine group participants during long-term follow-up compared with the placebo group in the Base Study, the incidence per 10 000 person-years of external genital warts related to HPV6 or 11 was 0·0 (95% CI 0·0–8·7) versus 137·3 (83·9–212·1), of external genital lesions related to HPV6, 11, 16, or 18 was 0·0 (0·0–7·7) versus 140·4 (89·0–210·7), and of anal intraepithelial neoplasia or anal cancer related to HPV6, 11, 16, or 18 in MSM only was 20·5 (0·5–114·4) versus 906·2 (553·5–1399·5).
Compared with during the Base Study (ie, before quadrivalent HPV vaccine administration), during the long-term follow-up period, participants in the catch-up vaccination group had no new reported cases of external genital warts related to HPV6 or 11 vs. 0 cases per 10 000 person-years or external genital lesions associated with HPV6, 11, 16, or 18 vs. 0 cases per 10 000 person-years, and a lower incidence of anal intraepithelial neoplasia or anal cancer related to HPV6, 11, 16, or 18 vs. 101·3 cases per 10 000 person-years.
Furthermore, there were no vaccine-related serious adverse events reported.
Gardasil is endorsed by the WHO, which says HPV vaccination prevents certain cancers by preventing infection by various HPV types.
This study was funded by Merck Sharp & Dohme, the producer of the Gardasil HPV vaccine.
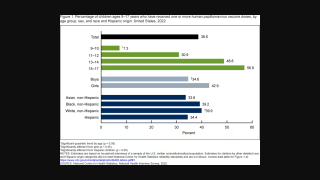
In the U.S., the American Cancer Society's updated recommendations are for healthcare providers to routinely offer the HPV vaccine series to boys and girls between ages 9 and 12.
Furthermore, the Gardasil 9 vaccine is offered in the U.S. It consists of HPV proteins, Types 6, 11, 16, 18, 31, 33, 45, 52, and 58.
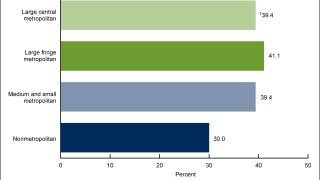
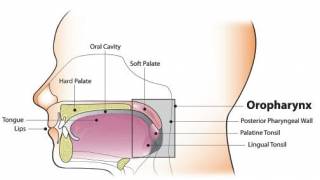
HPV infections can lead to certain cervical cancers. Many females with cervical cancer were probably exposed to cancer-causing HPV types in their teens and early 20s. Additionally, males can get HPV, causing anal and throat cancers and genital warts.
Our Trust Standards: Medical Advisory Committee