Initial HPV Vaccine Reduced Diseases in Women and Men

Merck announced yesterday that an updated systematic literature review examining the global impact and effectiveness of HPV vaccination using GARDASIL was published in the journal Expert Review of Vaccines.
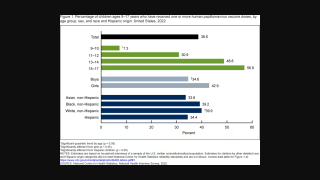
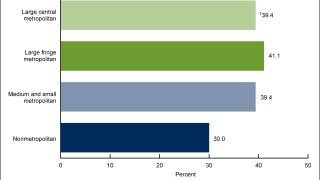
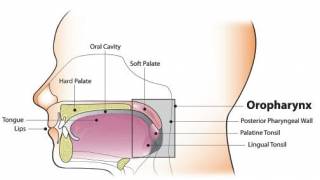
This systematic review excluded GARDASIL 9® and observed that the use of GARDASIL led to reductions in the rates of high-grade and low-grade cervical lesions and certain non-cervical HPV-related diseases and HPV infections in women and men.
“Since its first authorization in 2006, GARDASIL has been widely used in national immunization programs all around the world, which has enabled researchers to study the impact of HPV vaccination in a broad range of populations and settings,” said Ravinder Dhawan, vice president, head of the center for observational and real-world evidence, Merck Research Laboratories, in a press release on November 7, 2022.
“These real-world data from more than one hundred published studies show decreases in vaccine-type HPV infections and related diseases and are an important reminder that we need to do more to expand vaccination to males and females as part of the global fight to lessen the incidence of certain HPV-related diseases and cancers.”
The systematic review included 138 peer-reviewed studies published between March 2016 and 2020.
It details the impact and effectiveness of GARDASIL through immunization programs in 23 countries across Africa, Asia, Europe, Australia, South America, and North America. It builds on a prior review of real-world data published in 2016.
This review did not include studies examining GARDASIL 9® (Human Papillomavirus 9-valent Vaccine, Recombinant).
Note: GARDASIL is no longer marketed in the U.S. However, GARDASIL 9 was approved in the U.S. in 2014 and remains today a preferred cancer prevention vaccine.
Other HPV vaccine news is posted at PrecisionVaccinations.com/HPV.
Our Trust Standards: Medical Advisory Committee