Another Malaria Vaccine Approved in Africa
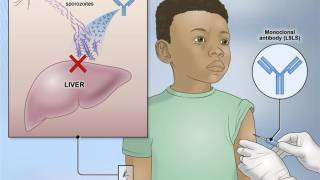
The University of Oxford today announced the R21/Matrix-M™ malaria vaccine was licensed for use in Ghana by the country’s Food and Drugs Authority.
This announcement marks the first regulatory clearance for the R21/Matrix-M malaria vaccine for use in any country for children at the highest risk of death from malaria.
According to the press release on April 13, 2023, the R21/Matrix-M vaccine has demonstrated high levels of efficacy and safety in Phase II trials, including amongst children who received a booster dose of R21/Matrix-M at one year following a primary three-dose regime.
The R21/Matrix-M malaria vaccine is a low-dose vaccine that can be manufactured at scale and modest cost, enabling as many as hundreds of millions of doses to be supplied to African countries which are suffering a significant malaria burden.
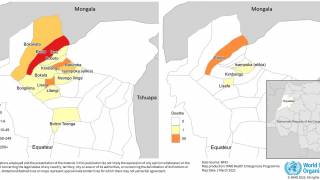
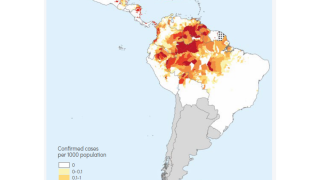
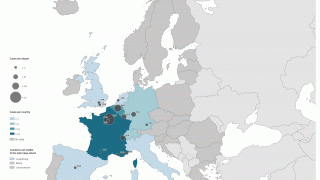
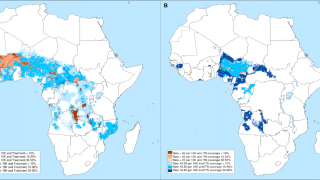
According to the U.S. Centers for Disease Control and Prevention, malaria is a vaccine-preventable mosquito-borne disease caused by a parasite. Malaria vaccines like Mosquirix™ and R21 have been reported effective at preventing disease.
As of April 13, 2023, these malaria vaccines are unavailable in the U.S.
Our Trust Standards: Medical Advisory Committee