Malaria Vaccine With Adjuvant Found Very Effective
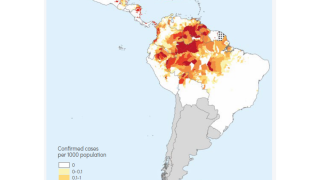
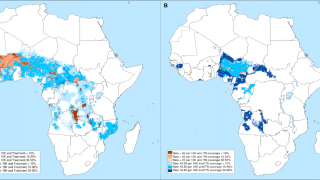
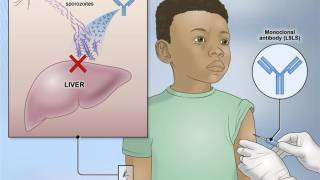
Malaria continues to be the most significant cause of death in young African children, with over 600,000 deaths globally each year. A recently approved malaria vaccine has been proven safe and effective to reduce this global health risk.
The Lancet today published peer-reviewed results from a Phase 3 efficacy clinical trial of the R21/Matrix-M™ malaria vaccine.
The publication reported on February 1, 2024, the following findings:
- Efficacy of 75% when administered before the high transmission season: In areas with highly seasonal malaria transmission (where malaria transmission is primarily limited to 4 or 5 months per year), the R21/Matrix-M vaccine was shown to reduce symptomatic cases of malaria by 75% during the 12 months following a 3-dose series.
- Efficacy of 68% when administered in an age-based schedule in regions where malaria is present perennially during the 12 months following the first three doses.
- Significantly increased immune responses to the R21/Matrix-M vaccine and slightly higher vaccine efficacy were observed in 5-17-month-olds supporting planned vaccine deployment initially from 5 months of age in young African children.
- The most common adverse events with the vaccine were fever (47%) and injection site pain (19%).
John Jacobs, Novavax President and CEO, commented in a press release, "Approximately 1,300 children die from malaria every day, a staggering statistic for a preventable disease."
"The R21/Matrix-M Phase 3 efficacy data published in The Lancet reinforce the potential of R21/Matrix-M vaccine to protect children against this disease."
"We are proud of the role of Novavax's patented saponin-based Matrix-M adjuvant, which has been demonstrated to enhance the immune response, in the outcome of this clinical trial and are eager to see the realized impact of the vaccine when it is rolled out globally."
The study was conducted across multiple sites in four African countries with 4,800 children aged 5-36 months. Data from this trial served as the basis for the World Health Organization's (WHO) recent prequalification of the R21/Matrix-M vaccine, paving the way for a global rollout expected to commence in mid-2024 by Serum Institute of India.
Ghana, Nigeria, Burkina Faso, and regulators in other countries have licensed the vaccine.
Beginning in February 2024, about 25 million R21/Matrix-M vaccine doses will become available. The availability of the R21/Matrix-M vaccine is expected to help close the gap in the vast demand for malaria vaccine doses to protect children against the disease.
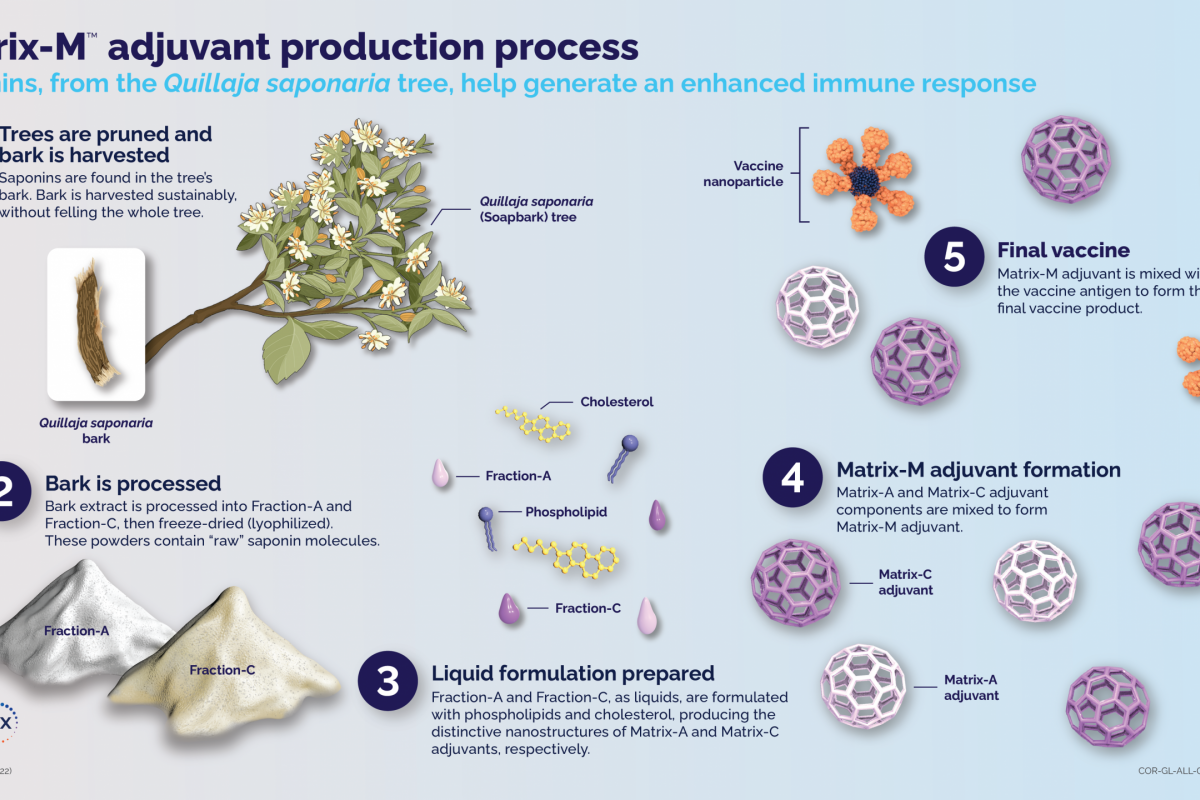
This innovative vaccine contains Novavax Inc.'s saponin-based Matrix-M™ adjuvant.
The Matrix-M adjuvant comes from saponins, naturally occurring compounds in the bark of the Quillaja saponaria (Soapbark) tree. Saponins have a long history of being used for their medicinal properties. The U.S. Food and Drug Administration has approved a vaccine containing another saponin‐based adjuvant.
Our Trust Standards: Medical Advisory Committee