$10 Million Advances Next Generation UTI Treatment

The global search for innovative urinary tract infection (UTI) treatments recently received a $10 million boost.
Recce Pharmaceuticals Limited, a leading Australian developer of a new class of Synthetic Anti-Infectives, announced it had received binding commitments to raise about $10 million in new funds.
On July 2, 2024, Recce confirmed that the funds raised from the Placement will be used to advance clinical trials for intravenous use of R327 and topical applications of R327G, including Registrational Phase III clinical activities in Indonesia, Investigational New Drug (IND) enabling activities, working capital, and offer costs.
In a press release, Chief Executive Officer James Graham commented on the capital raising: "We are" delighted with the support of our capital raising from our existing shareholders and welcome new institutional shareholders to our register. We thank NorthStar Impact Fund for taking the time to understand Recce and the positive impact we aim to achieve."
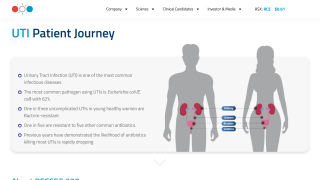
Recce stated the Company will be fully funded through to FY2026 to fund significant IND-enabling clinical trials in Australia, covering intravenous and topical treatments for UTI/Urosepsis and ABSSSI, including Diabetic Foot Infections, as well as U.S. Department of Defence Burn Wound Program, Continued development of a pre-clinical portfolio, manufacturing expansion and provides the necessary capital to see Indonesian clinical trials for topical treatments through to commercialization.
On July 1, 2024, Recce clarified the Phase I/II clinical trial is an Open Label, Adaptive Design Evaluation, Crossover Study of the Safety, Pharmacokinetics and Pharmacodynamics of Various RECCE® 327 (R327) Intravenous Dose and Infusion Rates.
The primary trial outcomes were to evaluate the safety and tolerability of R327 administered at various infusion rates ranging from 15 to 45 minutes in healthy male and female participants, and to assess the plasma pharmacokinetics of R327 using the same infusion rates.
The secondary trial outcomes focused on evaluating the concentration of R327 in urine at various doses and infusion rates and examining the ex vivo pharmacodynamics, specifically the minimum inhibitory concentration, of urine and blood samples from participants. Trial outcomes were successfully achieved.
An independent data review has been conducted, and the positive safety and efficacy conclusions stated in the announcement released on June 28, 2024. A comprehensive data review will be conducted, with results to be made available to the Company, which are expected to align with findings to date.
In the United States, And in the United States, Pivya™ (pivmecillinam) recently gained the Food and Drug Administration (FDA) approval for treating adult women with uncomplicated UTIs. Pivya's availability in the U.S. is forecasted for 2025.
Our Trust Standards: Medical Advisory Committee