Universal Flu Shot Candidate Granted Patent Protection

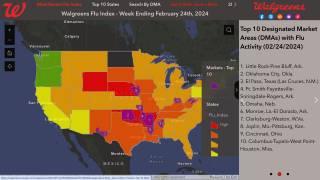
With the 2024-2025 influenza season underway in the United States and cases of swine flu being reported in people attending county fairs, an innovative vaccine candidate may soon solve these severe health issues.
Emergex Vaccines Holding Limited recently announced that it received patent protection from the United States Patent and Trademark Office (USPTO) for its novel class of influenza vaccines.
This USPTO patent covers Emergex’s vaccine candidate, which comprises immunogenic peptides encoded by a negative sense open reading frame (ORF) from segment 8 of the influenza A genome. This vaccine can potentially provide long-term T-cell immunity against legacy strains of influenza A, seasonal variants, and heterosubtypic changes.
To Emergex’s knowledge, this represents the first known patent for viral peptides derived from antigenomic translation, suggesting that segment 8 of influenza A is ambisense (negative and positive sense ORFs).
In addition, this grants the company exclusive rights to develop a vaccine that incorporates these immune elements, offering a level of immune recognition that existing flu vaccines cannot provide because of composition or method of administration.
Additionally, incorporating avian—and equine species-specific NEG8-derived peptides in a vaccine can potentially expand protection against zoonotic transmissions.
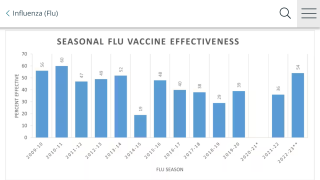
This is an essential feature of a universal flu shot. As influenza viruses change from year to year, influenza vaccines must be updated annually to include the viruses that will most likely circulate in the upcoming season.
Professor Thomas Rademacher, Co-Founder and Chief Executive Officer at Emergex, commented in a press release on August 22, 2024, “Our research into NEG8 has revealed exciting potential for a new approach to influenza vaccines. We believe that a vaccine composition including conserved NEG8-derived MHC class I peptides could protect against past, existing, and emerging human influenza viruses and prevent zoonotic influenza viruses from establishing themselves in the human population and causing a pandemic."
Emergex is set to advance its first-in-class influenza vaccine into the clinic, with Phase I trials anticipated to begin in the first half of 2025.
As of August 25, 2024, various U.S. FDA-approved cell, egg, and nasal flu shots are readily available at health clinics and pharmacies in the U.S.
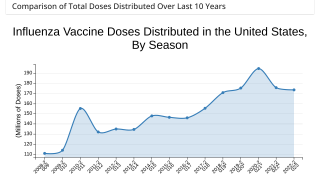
Over 157 million flu shots were distributed during the 2023-2024 season.
In the U.S., flu vaccines for the 2024-2025 season will be trivalent, and most (91%) will be thimerosal-free or thimerosal-reduced vaccines. About 21% of flu vaccines will be egg-free.
Our Trust Standards: Medical Advisory Committee