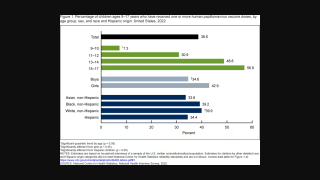
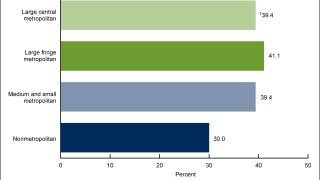
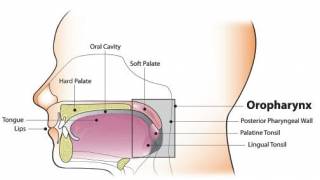
Vaccination Protects Japanese Men from HPV Cancers

Merck today announced positive top-line results from its pivotal Phase 3 clinical trial evaluating the company’s 9-valent Human Papillomavirus (HPV) vaccine, GARDASIL ® 9 vaccine, in Japanese males ages 16 to 26.
This trial (V503-064) met its primary and secondary endpoints demonstrating that administration of a 3-dose regimen of GARDASIL 9 reduced the combined incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
“A decade after the first approval of GARDASIL 9, Merck continues to evaluate this important vaccine in additional patient populations and remains committed to helping prevent certain HPV-related cancers through broad and equitable access globally,” said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories, in a press release on September 11, 2024.
“These data build on the clinical efficacy of GARDASIL 9 for the prevention of persistent infection in males and can potentially make a significant impact in addressing the global burden of certain HPV-related cancers and diseases.”
This HPV vaccine is generally available at clinics and pharmacies in the U.S. However, Merck says the GARDASIL 9 vaccination may not protect all vaccine recipients.
Our Trust Standards: Medical Advisory Committee