$41.9 Million Scales Up Ebola Treatments

While there is an approved Ebola virus disease (EVD) vaccine, the U.S. government continues to invest in human monoclonal antibody (mAb) therapy during Zaire ebolavirus outbreaks in Africa.
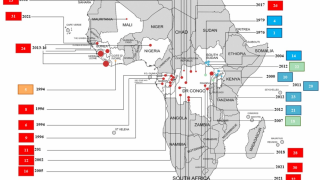
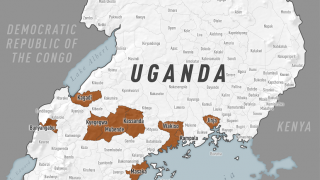
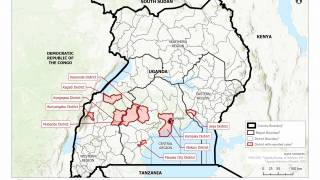
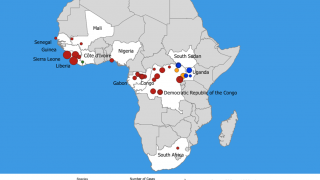
The initial Zaire Ebolavirus disease (EVD) case was confirmed in 1976, Since then, more than 30 EVD outbreaks have been reported.
Emergent BioSolutions Inc. announced today that it was awarded a contract modification executing an option period by the Biomedical Advanced Research and Development Authority (BARDA), valued at $41.9 million, for drug substance engineering and scale-up process validation, long-term stability, and commercial readiness in support of its ongoing scale-up program for Ebanga™, a licensed glycoprotein (EBOV GP)-directed mAb treatment for EVD.
“Emergent is proud to continue to advance the Ebanga™ (ansuvimab-zykl) development and scale up to its next phase,” said Paul Williams, senior vice president of products business, Emergent, in a press release on September 12, 2024.
This mAb binds to a portion of the Ebola virus's surface called the glycoprotein, which prevents the virus from entering a person's cells. Ebanga's efficacy has not been established for other species of the Ebolavirus and Marburgvirus.
Under the terms of the contract, Emergent will complete activities to advance the development of Ebanga™ treatment through post-licensure commitments, including the transfer of technology as part of manufacturing scale-up, submission of a supplemental Biologics License Application to the U.S. Food and Drug Administration (FDA), and completion of stability studies.
The existing 10-year contract consists of a base period of performance with two option periods for advanced development valued at approximately $121 million and option periods for procurement of Ebanga™ treatment over five years valued at up to $583 million. Execution of this option period is in line with Emergent’s planned program performance and critical path for developing the Ebanga™ treatment.
BARDA is part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
Our Trust Standards: Medical Advisory Committee