Oral Cholera Vaccine Approved in Canada

Bavarian Nordic today announced the commercial availability of Vaxchora® (CVD 103-HgR), the only single-dose oral vaccine approved in Canada to protect against cholera.
For approximately 10% of people infected with cholera, a sudden and severe intestinal infection marked by acute watery diarrhea, a severe presentation of cholera can rapidly cause dehydration and death if left untreated.
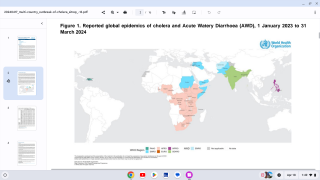
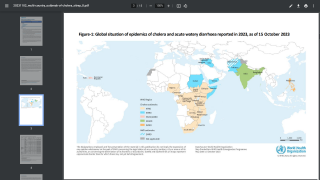
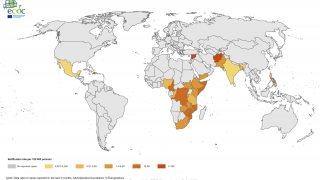
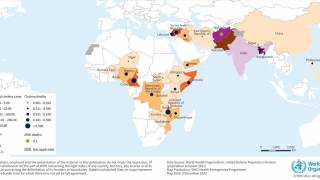
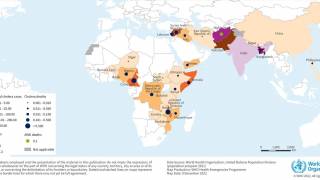
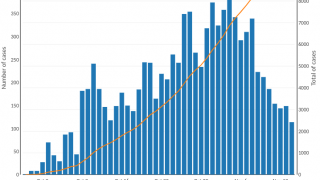
Nearly 4 million cases of cholera occur during outbreaks annually. The number of reported cholera-related deaths increased by 71% in 2023 compared to 2022.
“To help (Canadians) prevent illness while traveling internationally, we are expanding our vaccine offerings to include protection against cholera. Healthcare providers can now offer this new vaccine option to travelers who plan to visit countries where cholera is present,” said Karinne Lacombe, Canada Country Director, Bavarian Nordic, in a press release on September 16, 2024.
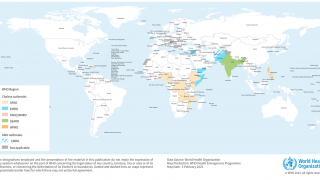
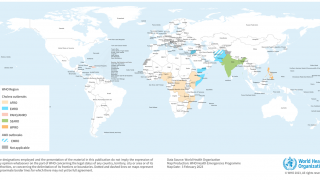
Cholera is endemic to approximately 50 countries and is common in Asia, Africa, and Central and South America. The Southeast Asia region, which includes Bangladesh and India, has the largest populations at risk for cholera.
While cholera is most commonly transmitted by consuming contaminated water, it may also be acquired from eating raw or undercooked food, especially fish and shellfish.
Other cholera vaccines, such as Valneva SE's oral, inactivated DUKORAL® vaccine, will be available globally in 2024. According to the World Health Organization, two doses of Dukoral® provide protection against cholera for two years.
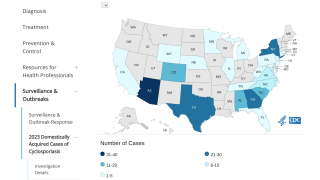
Most cholera cases in the United States are linked to international travel, such as to Haiti.
Travel vaccine experts offer cholera vaccination advice at clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee