Protein-based Vaccine Sales Strategy Advances

Novavax, Inc. today announced its financial results and operational highlights for the third quarter ended September 30, 2024. A few highlights are inserted below:
For the third quarter of 2024, total revenue was $85 million, compared to $187 million in the same period in 2023. And ended the quarter with $1 billion in cash and receivables.
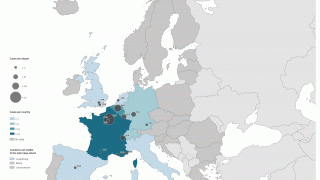
Advanced preparation for Sanofi to assume lead commercial responsibility of Nuvaxovid™ COVID-19 vaccine for the 2025-2026 vaccination season in the U.S., Europe, and select major markets not currently subject to Novavax Advanced Purchase Agreements or existing partnership agreements.
Entered the U.S. market with an improved Nuvaxovid presentation and broader access, available in pre-filled syringes in over 30,000 locations across major pharmacy retailers and regional grocers.
John C. Jacobs, President and Chief Executive Officer of Novavax, commented in a press release on November 12, 2024, “In addition to progress on our other value drivers, this past quarter, we made significant progress defining our R&D strategy as we look to expand beyond COVID-19 and influenza."
"We intend to develop our early-stage pipeline with a disciplined approach, as we focus on areas where our technology can have a positive impact on public health and generate value.”
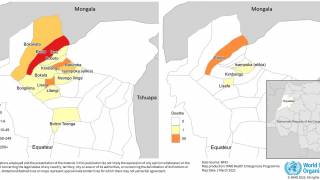
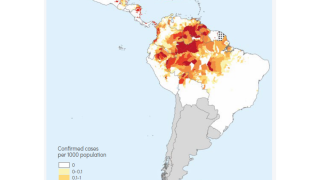
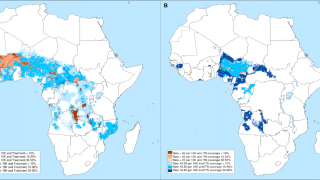
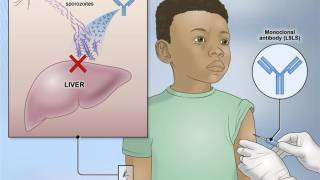
An example is the R21/Matrix-M™ vaccine that includes Novavax's proprietary saponin-based Matrix-M adjuvant. This malaria vaccine is manufactured by the Serum Institute of India Private Ltd and is available in various African countries in 2024.
Our Trust Standards: Medical Advisory Committee