HIV-1 Protein Nanoparticle Vaccine Candidate Reported Safe and Well-tolerated in Phase 1 Clinical Trial
Uvax Bio, LLC today announced the interim analysis results from its first Phase 1 clinical trial evaluating the Company’s HIV-1 vaccine candidates, UVAX-1107 and UVAX-1197.
In the first stage of this trial, the subjects received either UVAX-1107 adjuvanted with CpG 1018® and aluminum hydroxide or placebo.
UVAX-1107 was immunogenic and generated robust IgG responses to the vaccine antigen derived from an HIV-1 strain known as BG505. 100% of subjects in the vaccine group demonstrated antibody responses after two priming vaccinations with UVAX-1107.
Antibody response titers increased >200-fold 14 days after the 2nd dose compared to the same period following the first dose.
“We are pleased that our first Phase 1 trial is progressing smoothly, and we have preliminary confirmation that UVAX-1107 was well tolerated at all doses by the study participants, and no vaccine-related serious adverse events were reported,” said Pedro Garbes, M.D., Vice President and Global Clinical Lead of Uvax Bio, in a press release on November 19, 2024.
“As per the time of this 1st interim analysis, no participant was withdrawn from the study due to local/systemic reactogenicity; local and systemic adverse events were mild to moderate, transient, and resolved on average within two days. These preliminary safety results are aligned with expectations for an adjuvanted protein-based vaccine.”
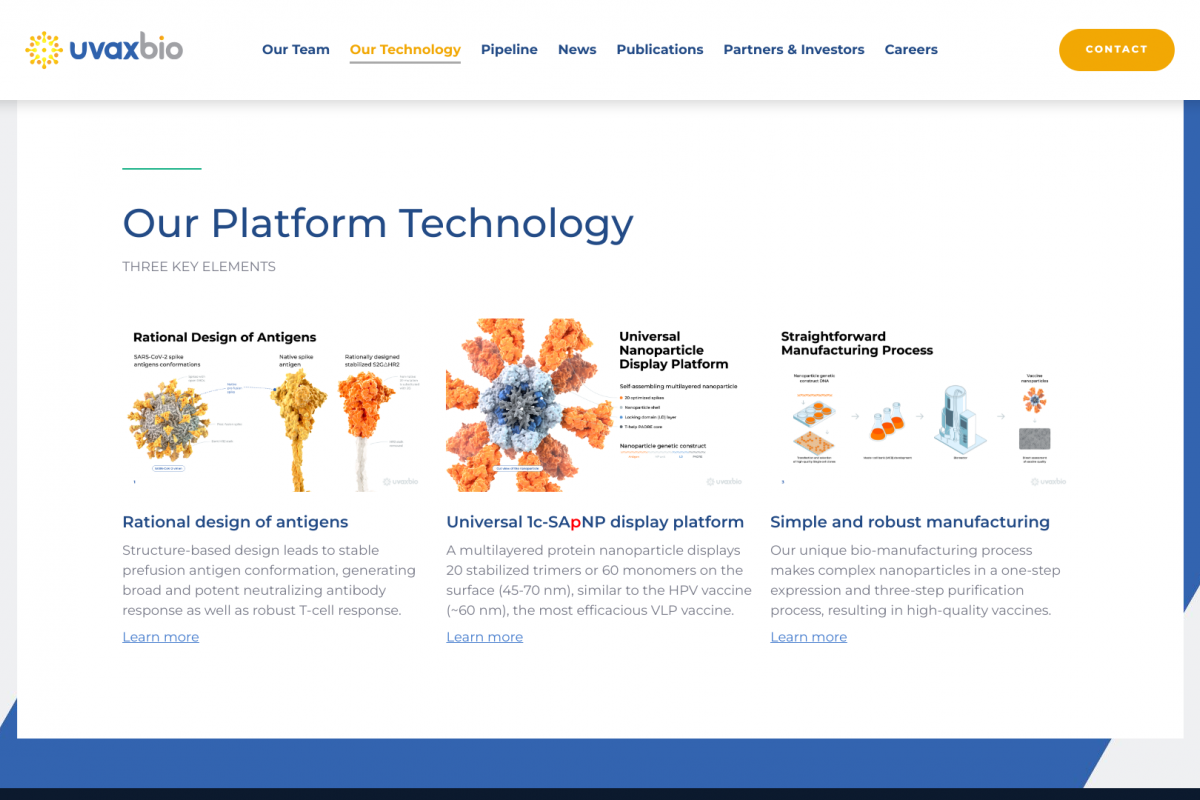
UVAX-1107 utilizes Uvax Bio’s 1c-SApNP® vaccine development platform to generate virus-like particles that closely resemble the target virus in size, shape, and multivalent antigen display; in this case, 20 copies of the native-like, prefusion-stabilized trimeric HIV-1 antigen.
Our Trust Standards: Medical Advisory Committee