mRNA Vaccines Targets HIV

Moderna Inc. recently announced it is advancing three Phase I clinical trials of Human immunodeficiency virus (HIV) vaccines with partners (mRNA-1644/IAVI G002; mRNA-1644/IAVI G003; mRNA-1574/NIAID) to expand on proof-of-concept data.
And these trials will evaluate the potential of Moderna's mRNA technology to successfully deliver immunogens and will be conducted in parallel to accelerate the advancement of immunogens into vaccine candidates.
The trials are the beginning of an iterative research process with the expectation for multiple Phase 1 trials to converge on a potentially protective vaccine that merits advancement to Phase 2.
These trials aim to determine whether this approach is safe and immunogenic, meaning that the immunogens elicit the correct type of broadly neutralizing HIV-1 antibodies (bnAbs).
A study published in February 2022 stated bNAbs targeting the HIV-1 envelope glycoprotein are promising molecules for therapeutic or prophylactic interventions.
Beyond neutralization, bNAbs exert Fc-dependent functions, including antibody-dependent cellular cytotoxicity and activation of the complement.
HIV, the cause of AIDS, is a sexually transmitted disease that has devastating health effects globally, resulting in approximately 650,000 related fatalities annually.
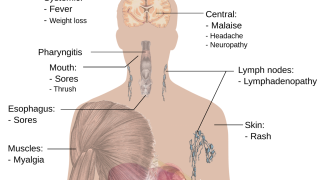
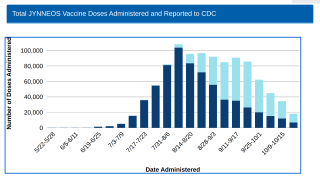
The U.S. Centers for Disease Control and Prevention recently identified a correlation between HIV and Mpox infections.
As of April 12, 2023, the U.S. Food and Drug Administration has not approved any HIV vaccine candidates for use in the United States.
Our Trust Standards: Medical Advisory Committee