HIV Vaccine Development Disrupted in Africa
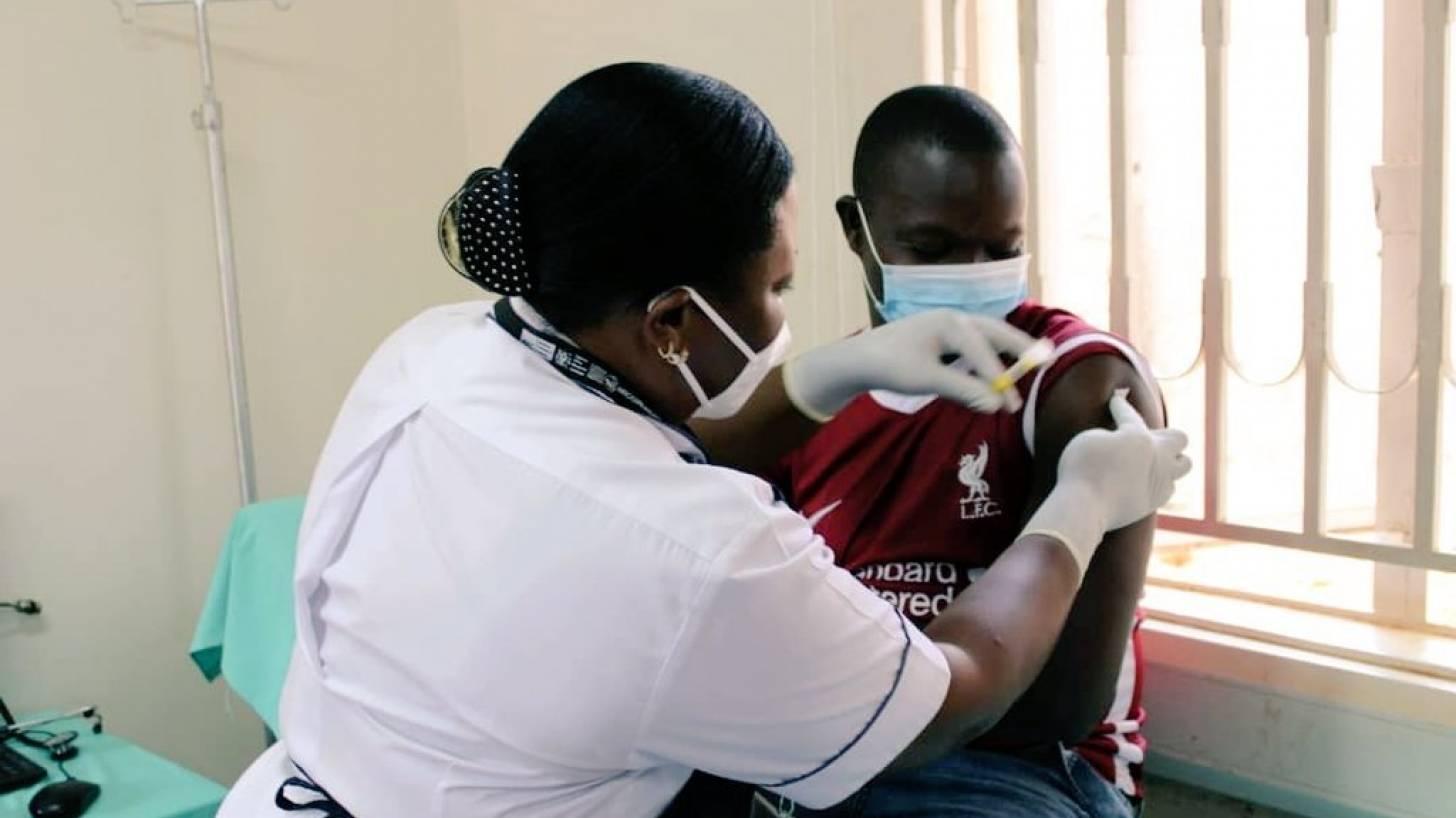
According to a recent announcement, a much anticipated human immunodeficiency virus (HIV) vaccine clinical trial that began in December 2020 has been discontinued in Africa because there is little or no chance of the trial demonstrating vaccine efficacy in preventing HIV acquisition.
On December 6, 2023, the PrEPVacc clinical trial leadership announced they decided to stop vaccinations based on the recommendation of its independent data monitoring committee (IDMC).
The PrEPVacc HIV prevention study of experimental vaccine regimens combined with a new form of oral pre-exposure prophylaxis (PrEP) was operating in East and Southern Africa among 1,500 volunteer participants.
However, the IDMC also recommended that the study's oral PrEP component (TAF/FTC, Descovy ©) continue to completion.
Results from this study are being analyzed and will be shared with participants, study teams, and the public in the second half of 2024.
This is unfortunate news, as there are no approved HIV vaccines as of December 11, 2023.
PrEPVacc's Trial Director, Dr. Eugene Ruzagira, who announced the news, commented in a press release, "Vaccinations to PrEPVacc trial participants have been stopped because an analysis of the data collected so far by our IDMC has led them to conclude that there is little or no chance of demonstrating that the vaccines we are testing are reducing the risk of acquiring HIV."
"The scientific hurdles are high, but I have equally high hopes that an HIV vaccine will be developed one day. Every day, important research like PrEPVacc is moving us forward worldwide."
"As we move towards a new era of HIV prevention studies and vaccine efficacy trials, the lessons of Good Participatory Practice have never been more important to apply."
PrEPVacc was three trials in one.
It was testing two different combinations of HIV vaccines to find out if either can prevent HIV infection in populations at risk of acquiring HIV.
Participants received injections of either one regimen combining a DNA vaccine with a protein-based vaccine (AIDSVAX), a regimen combining DNA, MVA, and a protein-based vaccine (CN54gp140), or a placebo.
Participants received four injections in each regimen or of the placebo.
At the same time as participants receive vaccinations, PrEPVacc is also testing a new oral PrEP drug formulation to see if it is as good as the drugs already approved for PrEP (TDF/FTC, Truvada©).
Participants received study PrEP as either Descovy © or Truvada © up to two weeks after the third vaccine injection. After that, the study teams either provide non-study PrEP in a clinic or refer the participants to access non-study PrEP at the local service providers.
The PrEPVacc study is funded by the European & Developing Countries Clinical Trials Partnership supported by the European Union.
Our Trust Standards: Medical Advisory Committee