$603,661 Funds Herpes Monoclonal Antibodies Optimization

The U.S. National Institute of Allergy and Infectious Diseases (NIAID) has funded a new study led by researchers at the Albert Einstein College of Medicine in New York with $603,661.
Launched on June 1, 2024, the goal of this follow-up project (PA-20-185) is to accelerate the development of strategies to bolster vaccine and monoclonal antibody (mAb) efficacy against a range of pathogens such as herpes simplex virus (HSV).
The total project funding amount for the two projects is now $1,231,761.
The NIAID says studies in mice and humans highlight the importance of antibody-dependent cell-mediated cytotoxicity (ADCC) in preventing HSV infections. This application aims to isolate and characterize monoclonal antibodies elicited by DgD-2, a novel candidate vaccine, with this protective activity and to define the role HVEM (herpes virus entry mediator) signaling pathways play in generating these IgG subclass switched ADCC antibodies.
As of June 5, 2024, no effective herpes vaccines or mAbs are available.
These researchers developed a novel single-cycle vaccine strain deleted in glycoprotein D (gD), designated DgD-2. DgD-2 completely protects mice from lethal challenges with clinical isolates of HSV-1 and HSV-2.
Protection is mediated by IgG2 antibodies that have little neutralizing activity but activate Fc gamma receptors to promote antibody-dependent cellular cytotoxicity (ADCC).
The central role of ADCC in mediating protection is supported by the observations that immune serum from DgD-2 vaccinated mice completely protects naïve wild-type, but not Fcg receptor IV knockout (FcgRIV-/-) mice, which is the primary receptor responsible for mediating ADCC in mice.
They isolated a highly protective mAb, BMPC-23, from DgD-2 vaccinated mice that exhibited potent FcgRIV activating activity and mapped its epitope to domain IV of viral glycoprotein B.
Notably, in contrast to the response to DgD-2, the humoral response to acute HSV infection or vaccination with gD subunit protein vaccines in both mice and humans is neutralizing with little or no ADCC. These observations suggest that gD may interfere with the elicitation of ADCC antibodies.
These researchers wrote, "We hypothesized that this novel immune evasion mechanism may be mediated by interactions between gD and the co-signaling molecule switch molecule herpes virus entry mediator (HVEM), expressed by most immune cells."
"When gD binds HVEM, the interactions between HVEM and its natural ligands (LIGHT, lymphotoxin-a, CD160, and BTLA) may be inhibited. Supporting our hypothesis, IgG2 ADCC responses were reduced, and protection against HSV was lost when Hvem−/− (knockout) mice were vaccinated with DgD-2."
"This need for HVEM to elicit IgG2 ADCC responses may be generalizable since similar results were obtained with other vaccines."
"Based on these data, we propose that the enhanced ADCC response to DgD-2 compared to natural HSV infection or gD protein vaccines reflects differences in viral targets recognized, IgG subclasses generated, and ability to overcome gD-HVEM mediated immune evasion."
"We will isolate and characterize mAbs generated in response to DgD-2, primary and recurrent HSV infection in wild-type compared to Hvem−/− mice to determine how HVEM signaling contributes to the antigenic repertoire, subclass and antibody function."
"We will test the mAbs that are generated in response to DgD-2 vaccination or infection individually and in combination (with BMPC-23 and other combinations) for their ability to protect and treat acute or recurrent HSV-1 and HSV-2 disease following vaginal and skin (male and female) infection as well as in models of neonatal disease. "
"Together, these studies will yield important new fundamental and translational knowledge applicable to the development of vaccines and mAbs that mediate ADCC," concluded these resarchers.
To address the lack of herpes vaccines or mAbs, the U.S. government issued a Notice of Special Interest on March 27, 2024, to stimulate additional focus on developing diagnostics, therapeutics, and vaccines targeting HSV.
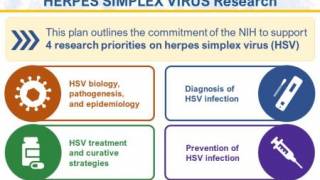
This Notice supports the U.S. NIH Strategic Plan for HSV Research 2023-2028, which focuses on HSV virology basic research, better HSV diagnostics, strategies to address HSV treatment and cure, and research to prevent HSV infection.
Our Trust Standards: Medical Advisory Committee