Herpes Prevention Has a Vaccine Development Plan

One of the challenges in treating Herpes simplex virus (HSV) infections is the diverse nature of clinical manifestations of the disease, wrote the U.S. National Institutes of Health in September 2023.
In many cases, symptomatic infection with HSV can lead to the development of herpetic lesions that present as cold sores or genital herpes, which are familiar sources of disease spread.
HSV infection predominantly occurs through contact with infected tissue of oral or genital mucosal surfaces, shedding the virus.
HSV can result in diverse and distinct pathology in other body tissues, including ocular keratitis, meningitis, or encephalitis.
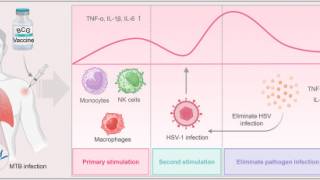
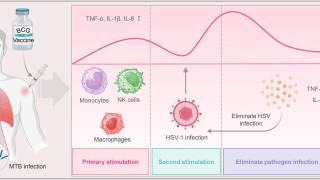
Once a "trigger" for transition out of latency occurs, HSV reverts into its active or lytic state. Known triggers for virus reactivation include stress, sunlight, immune suppression, and various other factors.
Recent studies have shown that live virus is shed without clinically evident classic herpetic lesions.
As such, developing and improving serologic and point-of-care diagnostics remains a research priority.
HSV infections are primarily treated with antivirals such as acyclovir (or valacyclovir), which can reduce the frequency and intensity of outbreaks of herpetic lesions if started early during the lytic cycle.
A prophylactic herpes vaccine that can prevent infection or a therapeutic vaccine for individuals already infected with HSV that could reduce recurrences and/or shedding would be crucial in mitigating the public health impact of herpes simplex viruses.
To address and coordinate efforts to facilitate HSV research, an NIH-wide HSV Working Group, led by the National Institute of Allergy and Infectious Diseases (NIAID), was established.
This group consists of scientific and policy experts from NIAID, the National Institute on Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, the National Institute on Minority Health and Health Disparities, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Eye Institute.
This effort aligns with the ongoing Sexually Transmitted Infections (Diseases) National Strategic Plan.
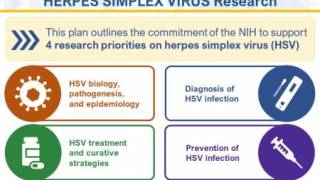
To advance NIH HSV research objectives, the NIH HSV Working Group organized this plan into four strategic priorities:
- improving fundamental knowledge of HSV biology, pathogenesis, and epidemiology;
- accelerating research to improve HSV diagnosis;
- enhancing strategies to treat and cure HSV and
- advancing research to prevent HSV infection.
These interdependent priorities form the framework of the NIH strategy to advance our understanding of HSV-1 and HSV-2 and help accelerate the development of innovative vaccines.
As of October 2023, there are several herpes vaccine candidates conducting clinical research.
These vaccines are based on DNA, modified mRNA, protein subunit, killed virus, and attenuated live virus vaccine technologies.
One vaccine candidate is Moderna Inc.'s mRNA-1608, which aims to induce a strong antibody response with neutralizing and effector functionality combined with cell-mediated immunity.
The mRNA-1608-P101 phase 1 clinical trial launched on September 6, 2023, and is forecasted to be completed in June 2025.
As of October 6, 2023, the U.S. Food and Drug Administration and agencies in Europe, Canada, China, India, and the U.K. had not authorized herpes prevention or therapeutic vaccines.
However, the NIH and global partners launched STI Watch, a portal containing updated information on vaccine development status.
Our Trust Standards: Medical Advisory Committee