Do Antibodies Help Vaccines Protect Against Herpes

A recent study conducted by researchers at Dartmouth's Geisel School of Medicine and Thayer School of Engineering has shed light on the role of antibodies in fighting herpes simplex virus (HSV) infections.
These findings, published in Cell Reports Medicine on February 12, 2024, could pave the way for new treatments or vaccines for neonatal herpes.
In their experiments, the research team used a neonatal mouse model to mimic human neonatal infections, engineering antibodies with different properties to explore how they mediate protection.
"Despite three decades of trying, the scientific community has been unable to develop an effective vaccine against herpes, and I think the main issue has been that we haven't fully understood what we need, in terms of antibodies and their specific functions, to protect against this disease," explains David Leib, chair and professor of microbiology and immunology at Geisel.
This research team found key differences between HSV-1 and HSV-2 infections and that each requires different antibody properties for optimal protection.
Importantly, their results point to a better way to design herpes vaccines and may help explain why many vaccine candidates have failed to be protective in clinical trials.
"Another important aspect of the work ....is that we now have some really good monoclonal antibodies (mAbs) that we've made in the lab that could potentially be used directly as a medication to treat acute neonatal herpes (nHSV) infections, which are life-threatening to newborns," says Leib.
"Monoclonal antibodies have been used to treat cancer and other diseases and show promise as a therapy for infectious disease," he says. "This would be a wonderful development, as antiviral drugs like acyclovir have only proven to be partially effective in treating these very sick kids."
These researchers investigated the mechanisms of mAbs-mediated protection in a mouse model of nHSV infection.
For a panel of glycoprotein D (gD)-specific mAbs, neutralization, and effector functions, they contributed to nHSV-1 protection.
When mAb was present at low concentrations, Abs with effector functions were more protective than Abs with neutralization activity alone. In contrast, neutralization activity was sufficient for high efficacy when present at high levels systemically or when pre-incubated with the virus before the nasal challenge.
This functional shift suggests that Ab concentration and biodistribution are determinants of the dominant protection mechanism.
In contrast, effector functions alone were sufficient to protect against nHSV-2, exposing a functional dichotomy between virus types consistent with vaccine trial results.
These researchers wrote that effector functions are therefore crucial for protection by these gD-specific mAbs, informing effective Ab and vaccine design and demonstrating the potential of polyfunctional Abs as therapeutics for nHSV infections.
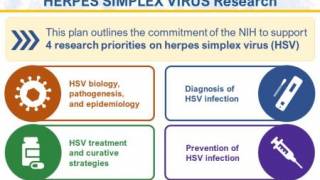
As of February 14, 2024, the U.S. FDA has not approved herpes prevention vaccines. To advance herpes vaccine research, the U.S. NIH recently established the Strategic Plan for Herpes Simplex Virus Research for 2023-2028.
Our Trust Standards: Medical Advisory Committee