Experimental Gene Editing Reduces 90% of Herpes Infections

The global gene editing market is forecast to reach around $29 billion by 2032, with the goal of targeting underserved diseases, such as herpes simplex virus (HSV).
Researchers recently announced that they found an experimental gene therapy for genital and oral HSV that removed 90% or more of the infection.
They developed a potentially curative approach against HSV infection based on gene editing using HSV-specific meganucleases delivered by adeno-associated virus (AAV) vectors.
Additionally, researchers at Fred Hutch Cancer Center located in Seattle, WA, stated that this pre-clinical therapy suppressed the amount of virus that can be released from an infected individual, which suggests that the therapy would also reduce the spread of the herpes virus.
Published in the journal Nature Communications on May 13, 2024, this experimental therapy involves injecting a mixture of gene-editing molecules into the blood to locate the herpes virus in the body.
The mixture includes laboratory-modified viruses called a vector — commonly used in gene therapies — plus enzymes that work like molecular scissors.
Once the vector reaches the clusters of nerves where the herpes virus hangs out, the molecular scissors snip away at its genes to damage them or remove the virus entirely.
"Herpes is very sneaky. It hides out among nerve cells and then reawakens and causes painful skin blisters," said Keith Jerome, MD, PhD, professor in the Vaccine and Infectious Disease Division at Fred Hutch, in a press release.
"Our aim is to cure people of this infection so that they don't have to live with the worry of outbreaks or transmitting it to another person."
The study's abstract says the gene editing performed with two anti-HSV-1 meganucleases delivered by a combination of AAV9, AAV-Dj/8, and AAV-Rh10 can eliminate 90% or more of latent HSV DNA in mouse models of orofacial infection, and up to 97% of latent HSV DNA in mouse models of genital infection.
Using a pharmacological approach to reactivate latent HSV-1, we demonstrate that ganglionic viral load reduction leads to a significant decrease in viral shedding in treated female mice.
While therapy is well tolerated, in some instances, we observe hepatotoxicity at high doses and subtle histological evidence of neuronal injury without observable neurological signs or deficits.
Simplifying the regimen through a single serotype (AAV9) delivering single meganuclease targeting a duplicated region of the HSV genome, dose reduction, and use of a neuron-specific promoter each results in improved tolerability while retaining efficacy.
These Fed Hutch researchers concluded these results reinforce the curative potential of gene editing for HSV disease.
Herpes simplex virus 1 and 2 are among the most common viral infections in the U.S., with up to 80% of people between the ages of 14 and 49 infected with HSV-1 and more than 10% infected with HSV-2, says the U.S. NIH.
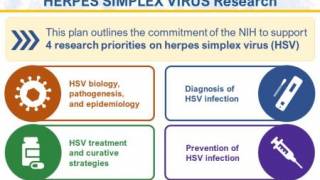
To advance research to understand and address HSV infection, the NIH has established the Strategic Plan for Herpes Simplex Virus Research. This plan aligns with ongoing national efforts, including the Sexually Transmitted Infections National Strategic Plan, and provides the framework for HSV research.
To date, no HSV vaccines have been approved by the U.S. FDA.
While herpes clinical trials advances have resulted in several therapeutics, the effectiveness of these treatments in reducing HSV symptoms and viral transmission varies widely.
Our Trust Standards: Medical Advisory Committee