Ebola Vaccines Embraced by Healthcare Workers
A recent study found that during the 2018–2020 Ebola virus disease (EVD) outbreak in the eastern part of the Democratic Republic of the Congo (DRC), healthcare workers (HCWs) were very receptive to taking an experimental vaccine.
On July 25, 2023, the journal Frontiers published Original Research that found about 99% of HCWs received the Ervebo® Ebola Vaccine (rVSV-ZEBOV-GP, rVSV-ZEBOV, v920) live, recombinant, replication-competent vaccine during the DRC's 10 Ebola outbreak.
Among the 438 HCWs offered vaccination, self-reported uptake of Ervebo was 99.0% (95% confidence interval [98.5–99.4]).
This research confirmed HCWs are at increased risk of EVD, given their close physical contact with patients. Nearly all HCWs (94.3%) perceived themselves at risk of contracting EVD.
Of the confirmed EVD cases during this Ebola outbreak in DRC, 5% were HCWs, and 44% died.
In 2019, Merck's Ziare EVD vaccine was licensed by the U.S. Food and Drug Administration for adults, followed by Canada and European authorizations.
On August 3, 2023, the FDA approved an expanded indication for ERVEBO for those 12 months and older.
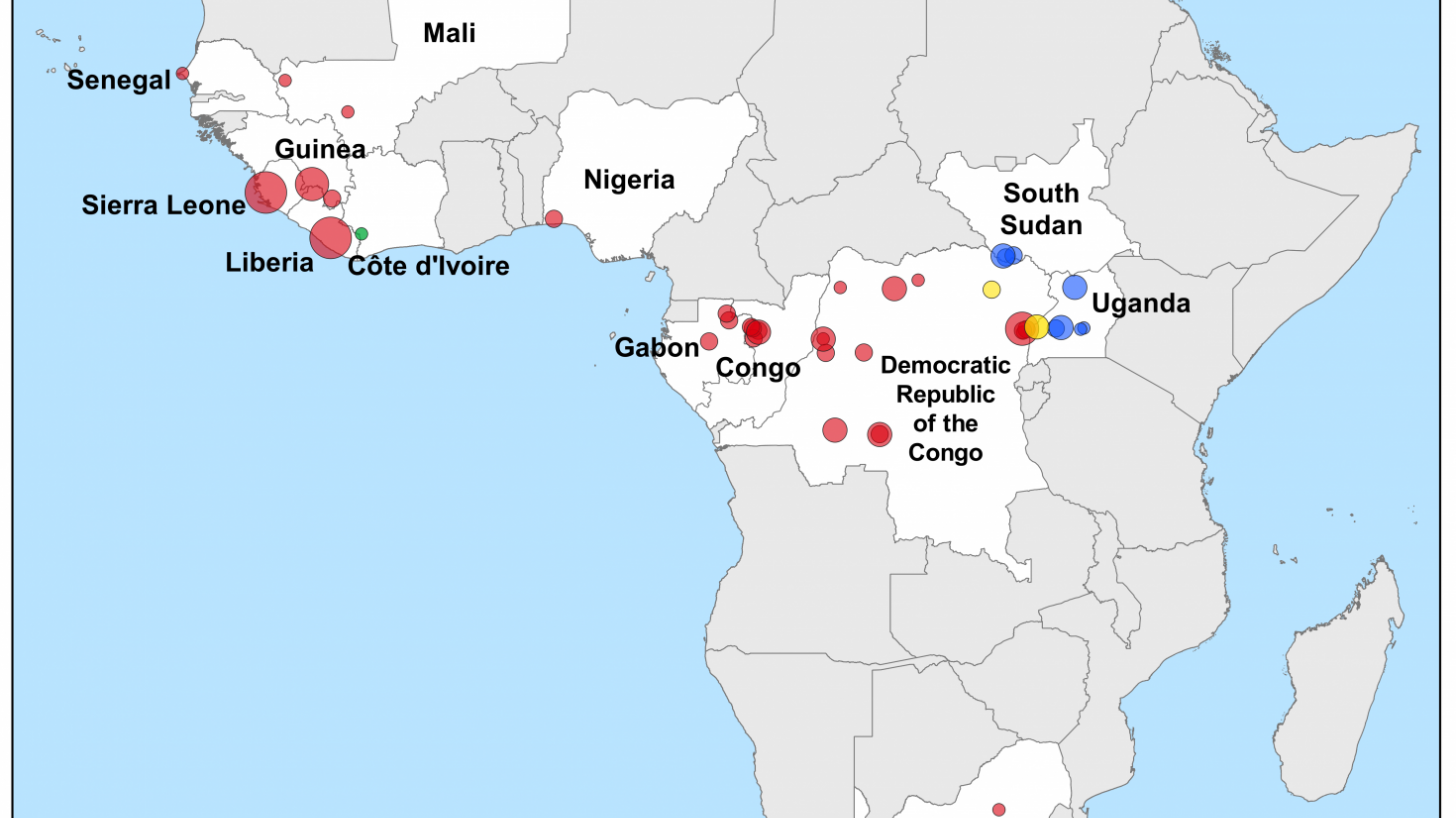
Zaire ebolavirus, Bundibugyo ebolavirus, and Sudan ebolavirus are the three species of ebolaviruses responsible for the more significant outbreaks of Ebola disease in Africa.
Zaire ebolavirus is the most fatal species. It was associated with numerous outbreaks and deaths in Africa.
Sudan ebolavirus, with a fatality rate of 50%, has been the cause of several outbreaks in Uganda and others near the border between South Sudan and DRC.
Bundibugyo ebolavirus, discovered in 2007, was associated with two outbreaks, one in DRC and the other on the border of DRC and Uganda.
And Taï Forest ebolavirus was the cause of one case identified in Côte d'Ivoire.
As of August 21, 2023, in certain countries, Ebola vaccines and antibody therapies are approved and offered. These products are of limited availability in the U.S.
Our Trust Standards: Medical Advisory Committee