Ebola Vaccination Recommended For At-Risk Healthcare Staff
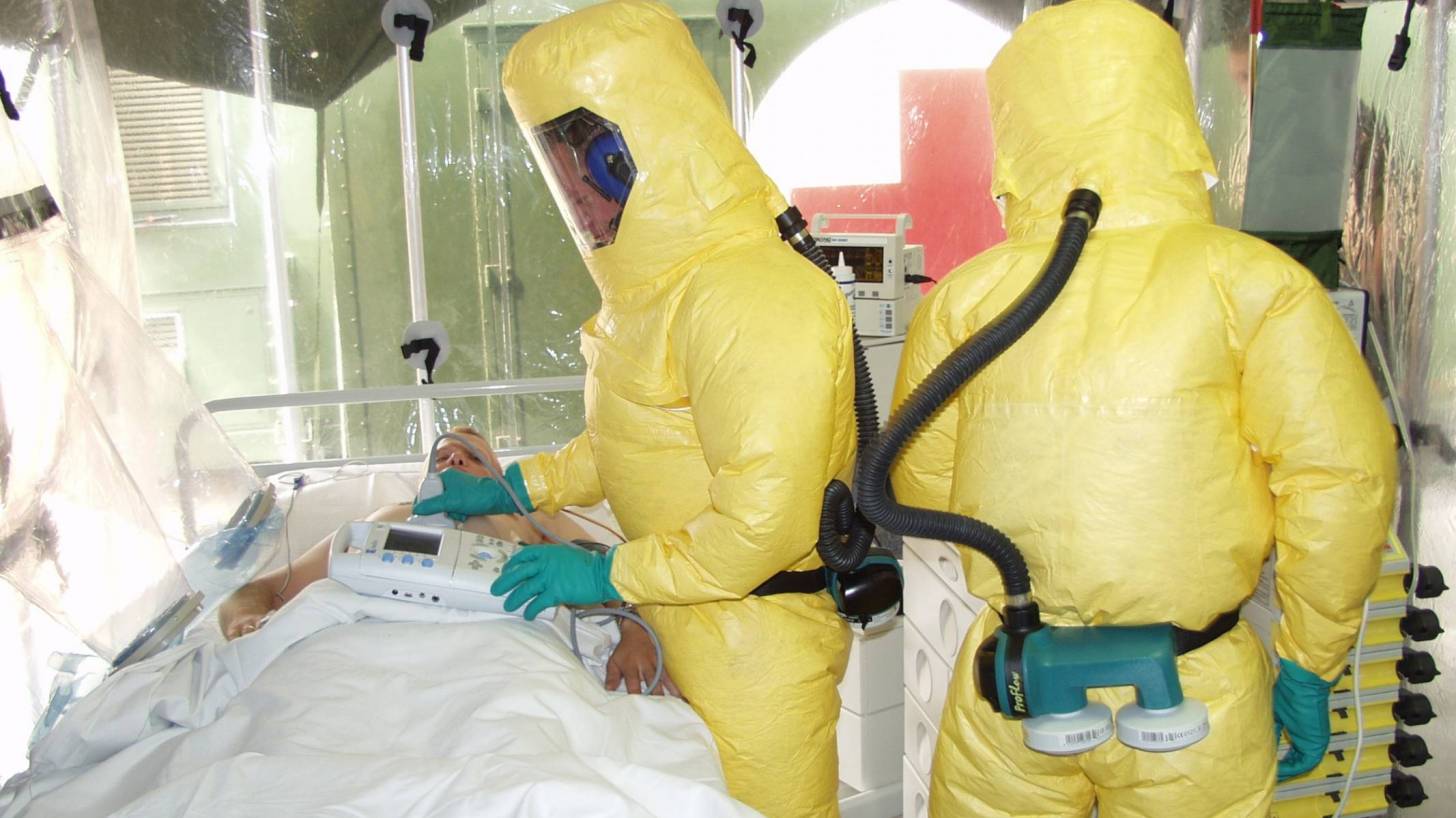
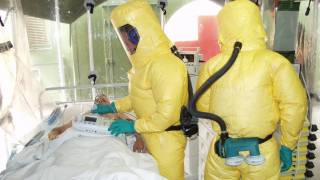
The committee responsible for vaccine recommendations has unanimously endorsed healthcare staff working in Ebola outbreak areas should be vaccinated with a newly approved vaccine.
The Advisory Committee on Immunization Practices (ACIP) recommended the use of the Ebola Zaire virus vaccine ERVEBO for certain U.S. residents who have an ‘occupational risk for exposure to the Ebola Zaire strain.’
Ervebo is a recombinant, replication-competent Ebola vaccine, consisting of a vesicular stomatitis virus, which has been genetically engineered to express a glycoprotein from the Zaire ebolavirus so as to provoke a neutralizing immune response to the Ebola virus.
This recommendation is now passed to the US Centers for Disease Control and Prevention (CDC) for the final review.
Additionally, U.S. military personnel also might require vaccination, according to Mary Choi, M.D., MPH, a medical officer at the National Center for Emerging and Zoonotic Infectious Diseases.
“The vaccine is indicated for the immunization of individuals 18 years of age and older to protect against Ebola virus disease caused by Ebola virus, Zaire Ebola virus species,” said Sharon E. Frey, M.D., who is on the Ebola vaccine working group of the ACIP.
The vaccine would be discretionary and based on the individual’s risk for exposure.
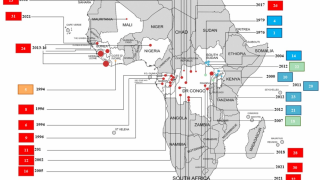
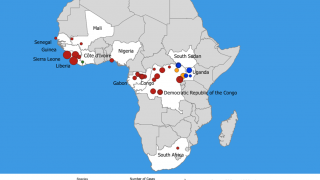
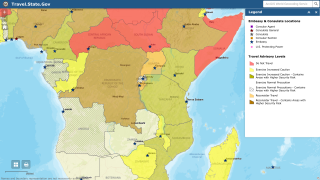
The number of Americans who would respond to an Ebola outbreak would vary. For instance, more than 4,000 government workers were deployed to the 2014-2016 West Africa EVD outbreak.
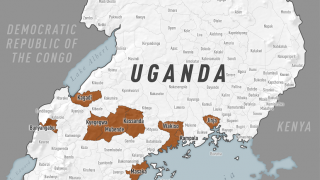
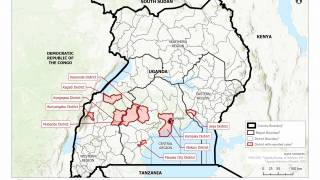
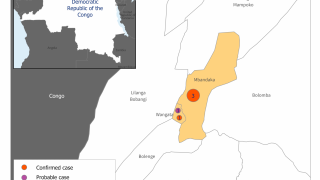
In the current outbreak in the Democratic Republic of the Congo (DRC), there are about 200 government personnel from the CDC, National Institutes of Health and U.S. Agency for International Development there, as well as about 200 people from nongovernmental agencies, according to Dr. Frey’s statement.
In addition, other staff who may not be directly involved in patient care, but who could be exposed to infectious agents might also receive this vaccine.
Among the pregnant women who received the vaccine in the trials, the frequency of pregnancy loss was higher in the vaccinated group. However, these data are difficult to interpret because there are few exposures and limited data on the outcomes of the pregnancies, and the timing of vaccination in relation to gestational age was difficult to establish.
In the United States, 11 people have been treated for EVD and all were related to the 2014-2016 West Africa outbreak: Nine were infected in West Africa, and 2 were cases of secondary transmission in Texas.
On February 15, 2020, the ERVEBO vaccine became registered by National Health Authorities in the Democratic Republic of Africa, Burundi, Ghana, and Zambia.
Ebola vaccine development news is published by Precision Vaccinations
Our Trust Standards: Medical Advisory Committee