Houston Has Prepared For Ebola’s Arrival
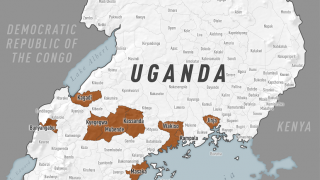
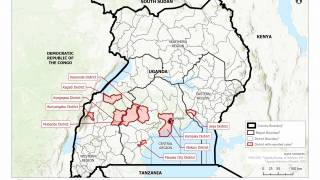
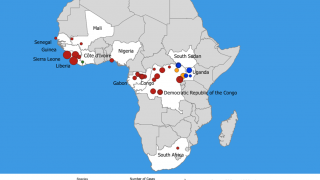
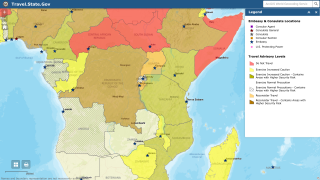
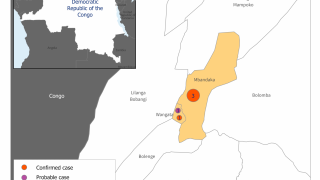
The ongoing Ebola Zaire outbreak in central Africa has spread from the Democratic Republic of Congo (DRC) to its neighboring country of Uganda.
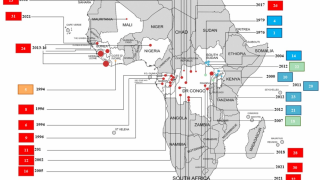
This Ebola outbreak in the DRC has left over 2,168 people infected and 1,440 dead since August 2018, according to the World Health Organization (WHO), as of June 15, 2019.
This WHO data represents a case fatality ratio of 66 percent.
In a statement on June 14, 2019, WHO Director-General Tedros Adhanom Ghebreyesus, Ph.D., said though the spread of Ebola virus disease (EVD) to Uganda is a new development, ‘it doesn't signal a shift in outbreak dynamics.’
A more significant outbreak risk is the potential international spreading of the Ebola virus, which happened previously.
During the DRC’s 2014 Ebola outbreak, 11 patients with EVD in total were treated in the United States, only 4 patients became ill after they arrived in the United States, either after exposure in West Africa or in a healthcare setting.
3 Ebola cases were reported in Dallas, Texas.
The initial case was the first travel-associated case of EVD diagnosed in the United States.
Two nurses who cared for the sick Ebola patient contracted EVD, marking the first known transmission of EVD in the United States, says the Texas Department of State Health Services (DSHS).
Unfortunately, the patient died, but both nurses recovered.
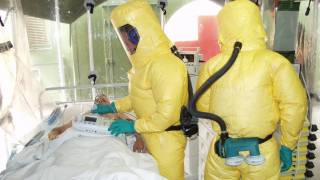
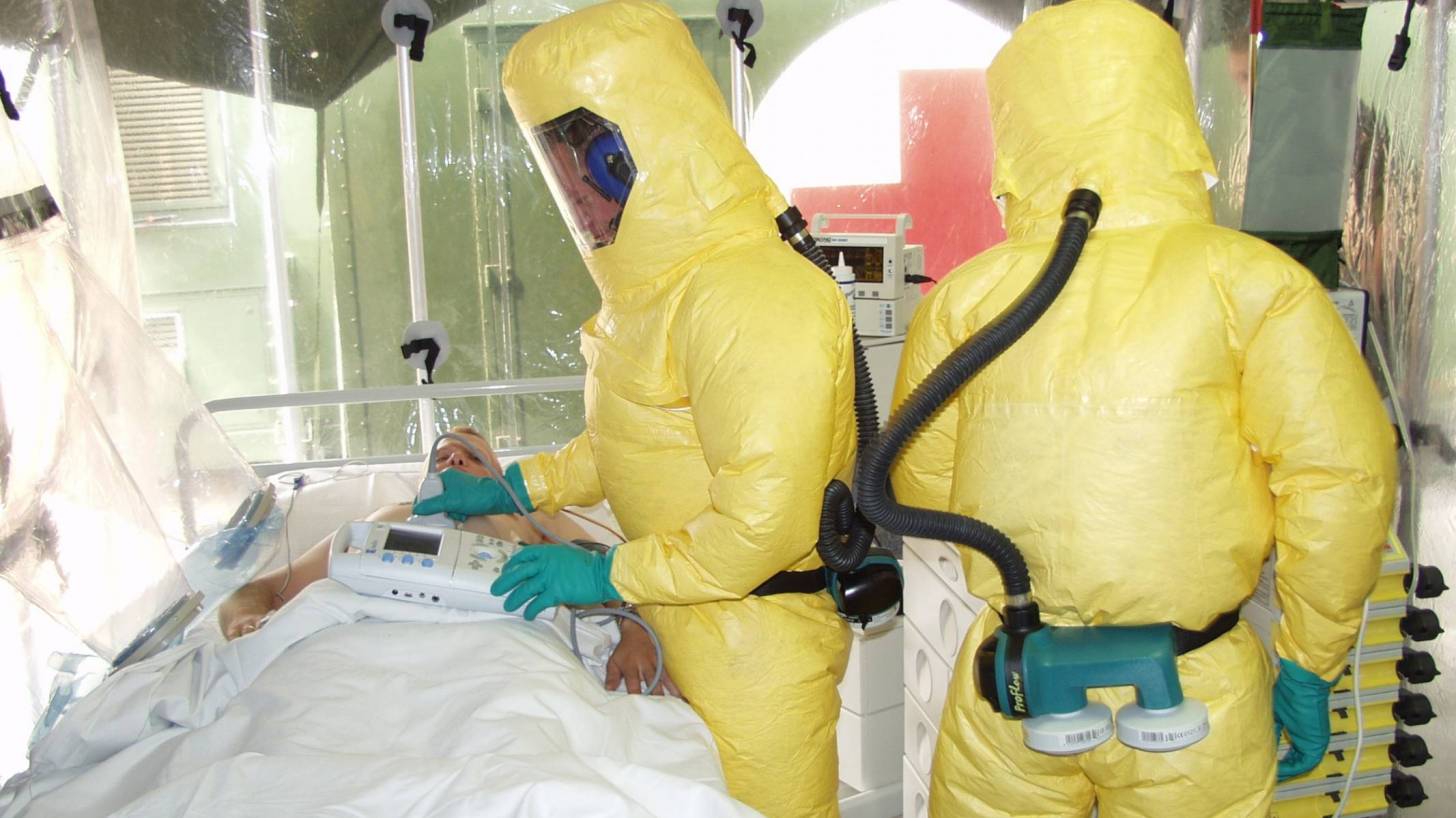
In response to these Ebola cases, the US federal government designated 55 hospitals as Ebola treatment centers.
Two Ebola-ready facilities are located in Houston, Texas, at The University of Texas Medical Branch in Galveston (UTMB) and Texas Children’s Hospital in Houston.
UTMB recently unveiled a state-of-the-art Biocontainment Care Unit specially designed to treat patients exposed to highly infectious diseases, such as Ebola.
The Galveston unit serves Texas, Louisiana, Oklahoma, Arkansas, and New Mexico.
“We’re part of a network of care providers who know how to do this and are willing to do this,” said Susan McLellan, M.D., Ph.D., medical director of UTMB’s Biocontainment Care Unit.
“The fact that we’re here, the fact that we think about it, means that we won’t have someone accidentally coming through the ER, being sent up to the general ward with something that could kill people.”
“This makes this kind of hospital the safest place to be in when there’s a risk of a highly infectious pathogen.”
The UTMB unit has been prepared to accept patients with Ebola and other infectious diseases since early 2015, the new unit has 6 patient rooms built with special surfaces and negative airflow to filter out infectious particulates, as well as to ensure patient and health care worker safety.
In West Houston, a second hospital is focused on treating children with Ebola.
At the Texas Children’s hospital campus in Katy, an entirely new unit was designed for highly infectious diseases. This care unit has 8 beds behind locked doors.
Designed specifically for children with highly contagious infectious diseases, such as Ebola and MERS, the unit allows the expert team to provide care to patients who are infected with communicable diseases in a state-of-the-art, safe environment.
“After the cases in Dallas, we knew there would be a need for specializing children’s care,” said Dr. Judith Campbell, medical director for infection control and prevention at Texas Children’s.
“We’re doing everything we can to make certain that if we see a case of Ebola we will not have secondary cases among health care workers or among the other patients and families we serve.”
While these Houston facilities are ready to treat Ebola patients, there is one preventive tool not yet in the ‘Ebola-prevention-tool-box.’
There is currently no vaccine licensed by the U.S. Food and Drug Administration (FDA) to protect people from the Ebola virus.
An experimental Ebola Zaire vaccine candidate called V920 (rVSV-ZEBOV) has been found to be highly protective (97.5%) against the virus in the DRC.
V920 vaccine has been administered to 135,395 people during this DRC outbreak.
The v920 vaccine is under study by the National Institutes of Health in an open-label clinical trial, entitled “Pre-Exposure Prophylaxis in Individuals at Potential Occupational Risk for Ebola Virus Exposure” or “PREPARE.”
The PREPARE study sites are located at the NIH in Bethesda, MD, and Emory University in Atlanta, GA.
But, the v920 vaccine appears to be in limited supply.
Merck, which makes the v920 (VSV-EBOV) vaccine, recently announced it has 250,000 doses ready to ship and expects to make 100,000 more by the end of 2019.
If Merck cannot meet demand, another Ebola vaccine candidate from Johnson & Johnson Ad26.ZEBOV/MVA-BN stands ready to support Ebola outbreak response efforts.
A significant stockpile of approximately 1.5 million of the Janssen investigational Ebola vaccine regimen is ready for potential deployment in public health emergencies.
Meanwhile, to aid Uganda in its Ebola response, the WHO flew in 4,000 doses of the v920 vaccine, according to a news Tweet by Uganda Minister of Health Dr. Jane Ruth Acen, on June 13, 2019.
“I want the entire nation (Uganda) to observe a no handshaking/body contact phase until we are Ebola-free,” Tweeted Dr. Acen.
Our Trust Standards: Medical Advisory Committee
- EVOLUTION OF THE EBOLA EPIDEMIC IN THE PROVINCES OF NORTH KIVU AND ITURI
- Statement on the meeting of the International Health Regulations (2005) Emergency Committee for Ebola virus disease in the DRC
- IHR Emergency Committee on Ebola in North Kivu, Democratic Republic of the Congo
- Texas Health and Human Services: Ebola
- UTMB’s new biocontainment care unit is prepared for the next Ebola outbreak
- West Campus: Special Isolation Unit
- Americans Can Benefit From Merck’s Ebola Vaccine
- Is San Antonio Prepared to Treat Ebola?
- CDC: Ebola in the United States