New Ebola Vaccine Approved For Use in the USA

A new vaccine for the prevention of Ebola virus disease, caused by Zaire ebolavirus, was approved by the U.S. Food and Drug Administration (FDA).
Announced on December 19, 2019, the Ervebo (v920) became the first FDA-approved vaccine for individuals 18 years of age and older.
The Ervebo vaccine is a live, recombinant, replication-competent Ebola vaccine, consisting of a vesicular stomatitis virus (VSV), which has been genetically engineered to express a glycoprotein from the Zaire ebolavirus, so as to provoke a neutralizing immune response to the Ebola virus.
The Ervebo vaccine is administered as a single-dose injection.
The safety of Ervebo was assessed in approximately 15,000 individuals in Africa, Europe, and North America.
Additionally, the Ervebo vaccine has been more than 250,000 times used under the 'compassionate use' protocol in central Africa since 2018.
The most commonly reported side effects were pain, swelling, and redness at the injection site, as well as headache, fever, joint, and muscle aches, and fatigue.
Merck & Co. Inc. licensed the global R&D and manufacturing rights for Newlink Genetics Corp.'s phase I Ebola vaccine. The Public Health Agency of Canada, which originally developed the vaccine, retained noncommercial rights in the agreement.
On November 11, 2019, the European Commission announced the granting of marketing authorization to Merck for its Ervbo vaccine.
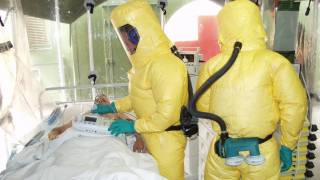
While Ebola virus disease (EVD) cases have been very rare in the USA, and those cases that have occurred, have been related to international travel and the result of infections acquired when treating an EVD patient.
Ebola virus can be detected in blood after the onset of symptoms. It may take up to three days after symptoms start for the virus to reach detectable levels. Polymerase chain reaction (PCR) is one of the most commonly used diagnostic methods because of its ability to detect low levels of the Ebola virus, says the US Centers for Disease Control and Prevention (CDC).
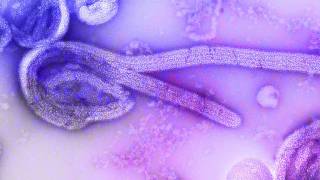
There are 4 known species within the genus Ebolavirus that are known to cause disease in humans: Ebola virus, Sudan virus, Bundibugyo virus, and Taï Forest virus.
Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, said in a related press release, “The FDA’s approval of Ervebo is a major advance in helping to protect against the Zaire ebolavirus as well as advancing U.S. government preparedness efforts.”
“The research approach used to study the effectiveness and safety of this vaccine was precedent-setting during a public health emergency and may help create a model for future studies under similar circumstances.”
The FDA granted this application Priority Review and a Tropical Disease Priority Review Voucher on November 14, 2019. The FDA also granted Breakthrough Therapy designation for Ervebo to facilitate the development and scientific evaluation of the vaccine.
Because of the public health importance of a vaccine to prevent EVD, the FDA worked closely with Merck and completed its evaluation of the safety and effectiveness of Ervebo in less than six months.
Recently, a CDC Health Advisory was published by the Alert Network Network (HAN) updated usage recommendations regarding the FDA approved OraQuick® Ebola Rapid Antigen Test.
Published on December 16, 2019, this Health Advisory (#432) says ‘healthcare providers caring for a patient with possible Ebola virus infection should first contact their local or state public health authorities before any testing is performed.’
The FDA is an agency within the U.S. Department of Health and Human Services, that protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices.
Ebola Vaccine news published by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee