Ebola Zaire Vaccine Candidate Rolls Forward

Merck announced that it has started the submission of a rolling Biologics License Application (BLA) for the Ebola Zaire disease vaccine candidate, V920 (rVSV∆G-ZEBOV-GP).
This rolling submission is made pursuant to the U.S. Food and Drug Administration (FDA)’s Breakthrough Therapy Designation for V920, which was announced by the company in July 2016.
Paula Annunziato, M.D., vice president for clinical research at Merck Research Laboratories said in a press release, “We are fully committed to the development of this important vaccine against Ebola.”
“In the meantime, pre-licensure, investigational doses of V920 are available to support response to Ebola Zaire outbreaks on an emergency basis in coordination with global public health authorities.”
In 8 Phase I and 4 Phase II/III clinical trials with approximately 17,000 subjects, V920 showed the immunogenicity was detectable at 14 days post-vaccination, and with up to 100% seroconversion observed by 28 days postdose.
In late 2014, when the Ebola disease outbreak in western Africa was at its peak, Merck licensed V920 from NewLink Genetics.
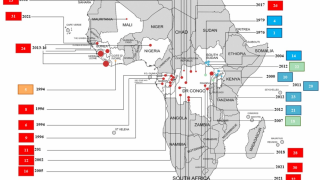
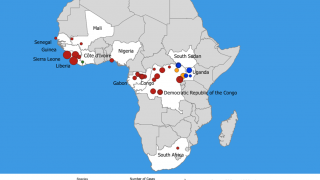
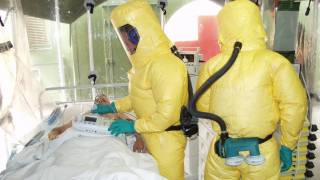
The Ebola Hemorrhagic Fever outbreak in West Africa during 2013–2016 was the first to impact both rural and urban areas, reported the Centers for Disease Control and Prevention (CDC).
As of October 29, 2018, the ‘rVSV-Ebola’ vaccine is not commercially licensed.
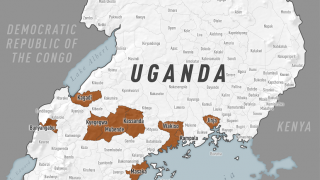
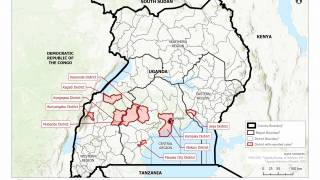
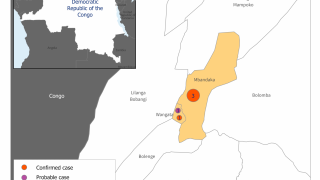
This vaccine candidate is being used under “expanded access” or what is also known as “compassionate use” in the ongoing Ebola outbreak in the Democratic Republic of Congo (DRC).
Currently, Merck expects the rolling submission of the BLA to be completed in 2019.
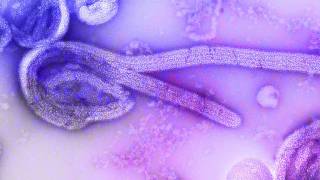
The Ebola virus is very contagious and is spread through inhalation, ingestion and or passage through breaks in the skin when an uninfected person comes in contact with blood or other body fluids, says the CDC.
Separately, Merck announced its ‘rVSV-Ebola’ vaccine candidate will be administered to the health workers to protect them against the type of Ebola virus strain that is currently circulating in some parts of DRC.
The FDA’s Breakthrough Therapy Designation is intended to expedite the development and review of a vaccine candidate that may demonstrate substantial improvement over existing therapies.
The V920 vaccine was initially engineered by scientists from the Public Health Agency of Canada’s National Microbiology Laboratory and subsequently licensed to a subsidiary of NewLink Genetics Corporation.
The company has committed to working closely with other stakeholders to accelerate the continued development, production and, if licensed, distribution of the vaccine.
For more than 80 years, Merck has contributed to the discovery and development of novel medicines and vaccines to combat infectious disease.
Our Trust Standards: Medical Advisory Committee
- Merck Begins Rolling Submission of Licensure Application for V920 (rVSV∆G-ZEBOV-GP) to U.S. Food and Drug Administration
- Merck Receives Breakthrough Therapy Designation from FDA and PRIME Status from EMA for Investigational Ebola Zaire Vaccine V920
- Ebola virus disease candidate vaccines under evaluation in clinical trials
- Ebola Vaccine Development Race Between the USA and China
- Uganda Prepares It’s Ebola Defense