Mpox Vaccine Found Safe for Children

A peer-reviewed study recently published by the Lancet Infectious Diseases assessed the safety, reactogenicity, and humoral and cellular immune response following the first reported use of the Bavarian Nordic JYNNEOS® (MVA-BN) vaccine in young children.
Published on June 16, 2023, this UK Health Security Agency-funded study concluded a single dose of MVA-BN for post-exposure prophylaxis was well-tolerated in children and induced robust antibody and cellular immune responses up to 15 weeks after vaccination.
Our findings support MVA-BN's ongoing use to prevent mpox in children as part of an emergency public health response. However, more extensive studies are needed to fully assess the safety, immunogenicity, and effectiveness of MVA–BN in children, wrote these researchers.
This is an important finding since a small amount of published literature indicates that children and pregnant women are at increased risk of mpox.
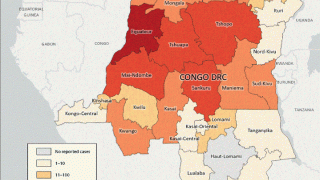
As of late 2022, there were 3547 mpox cases confirmed in England, including one child, a neonate.
This early evaluation study was conducted between June 1 and November 30, 2022, while 87 children about five years of age received one MVA–BN dose.
Post-vaccination reactogenicity questionnaires were completed by 45 children.
Thirty-six children reported no symptoms, 40% reported local reactions only, and 24% reported systemic symptoms with or without local reactions.
All children had poxvirus IgG antibodies with titres well above the assay cutoff with mean absorbances at six weeks and 15 weeks post-vaccination.
Assessment of reactivity to 27 recombinant vaccina virus and monkeypox virus proteins showed humoral antigen recognition, primarily to monkeypox virus antigens B6, B2, and vaccina virus antigen B5, with waning of humoral responses observed between the two-time points.
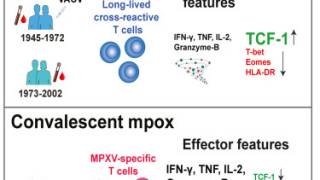
All children had a robust T-cell response to the whole modified vaccinia Ankara virus and a select pool of conserved pan-Poxviridae peptides.
A balanced CD4+ and CD8+ T-cell response was evident at six weeks, which was retained 15 weeks after vaccination.
Further studies are also needed to assess immune responses and duration after two doses, added these researchers.
On June 16, 2023, the UKHSA announced that mpox vaccinations would remain available after 11 new cases were recently diagnosed near London.
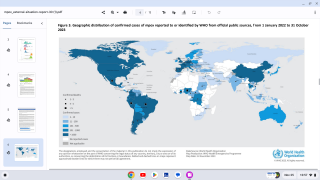
The World Health Organization recently confirmed around 275 new mpox cases per week.
New and breakthrough mpox cases have been reported in Belgium, Chicago (U.S.), Denver (U.S.), Netherlands, France, South Korea, Spain, Japan, Thailand, and Paraguay.
The MVA–BN (JYNNEOS) vaccine was developed with the U.S. Government to ensure adult populations can be protected from smallpox. It was approved by the U.S. Food and Drug Administration on September 24, 2019.
It is now indicated for preventing both smallpox and mpox diseases in adults.
MVA–BN has not been administered to children of any age in pre-licensure or post-licensure studies and is not licensed in children.
Our Trust Standards: Medical Advisory Committee