Mpox Human-to-Human Transmission Confirmed In African Countries

The Centers for Disease Control and Prevention (CDC) today issued Health Alert Network (HAN) Health Update CDCHAN-00513 to provide additional information about the outbreak of monkeypox virus (MPXV) in the Democratic Republic of the Congo (DRC) and neighboring countries.
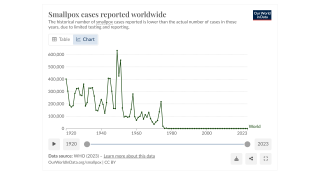
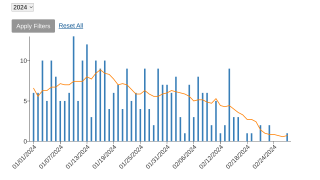
Since January 2023, the DRC has reported the most significant number of yearly suspected clade I mpox cases on record.
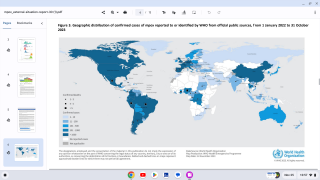
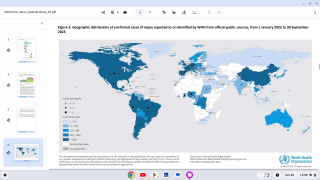
While clade I MPXV is endemic, or naturally occurring, in the DRC, the current outbreak is more widespread than any previous DRC outbreak. As of August 7, 2024, no cases of clade I mpox have been reported outside central and eastern Africa.
The Republic of the Congo (ROC), which borders DRC to the west, declared a clade I mpox outbreak in April 2024, and there have been confirmed cases in the Central African Republic (CAR).
While clade I mpox is endemic in ROC and CAR, the epidemiologic pattern of recent cases suggests a possible link to DRC.
In late July 2024, Burundi, Rwanda, and Uganda, which sit on the eastern border of DRC, reported confirmed cases of mpox, with some instances having linkages to DRC.
Rwanda and Uganda have confirmed that these cases are due to clade I MPXV; in Burundi, clade-specific testing is underway, but cases are presumed to be clade I due to the DRC’s proximity.
Mpox is not known to be endemic in these countries.
Because there is a risk of additional spread, CDC recommends clinicians and jurisdictions in the United States maintain a heightened index of suspicion for mpox in patients who have recently been in DRC or to any country sharing a border with DRC (ROC, Angola, Zambia, Rwanda, Burundi, Uganda, South Sudan, CAR) and present with signs and symptoms consistent with mpox.
These can include a rash that may be located on the hands, feet, chest, face, mouth, or near the genitals; fever; chills; swollen lymph nodes; fatigue; myalgia (muscle aches and backache); headache; and respiratory symptoms like sore throat, nasal congestion, and cough.
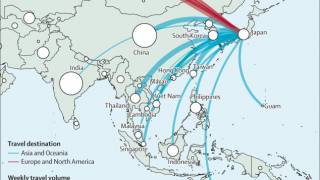
The CDC says due to the limited number of travelers and lack of direct commercial flights from DRC or its neighboring countries to the United States, the risk of clade I mpox importation to the U.S. is considered to be very low.
The CDC is helping communities monitor the presence of both clades of MPXV in wastewater samples, including from select airports. Data from samples can provide an early warning of mpox activity and spread in communities.
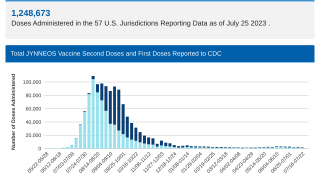
Additionally, healthcare providers should consider vaccinating patients eligible for mpox vaccination and planning travel to affected countries with two doses of the U.S. FDA-approved JYNNEOS vaccine.
Eligible patients who received one dose of Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®) mpox smallpox vaccine more than 28 days ago should receive the second dose as soon as possible.
JYNNEOS is available in health clinics and pharmacies in the U.S.
CDC’s Health Alert Network is the CDC’s primary method of sharing information about urgent public health incidents with public information officers, federal, state, territorial, tribal, and local public health practitioners, clinicians, and public health laboratories.
Our Trust Standards: Medical Advisory Committee