Influenza B Virus Causing 72% of Pediatric Fatalities in 2019-2020

According to the new Centers for Disease Control and Prevention (CDC) FluView report, there have been 16 pediatric fatalities associated with influenza B viruses during the 2019-2020 flu season.
Published on December 27, 2019, this FluView report confirmed 5 of these 16 fatalities had the lineage determined and all were B/Victoria viruses.
Additionally, 6 child deaths were associated with influenza A viruses. Four of these were related to A(H1N1)pdm09 viruses.
In total, the CDC reported there have been 22 pediatric fatalities confirmed as of December 21, 2019, which compares with 143 cases during the 2018-2019 flu season.
Nationally, influenza B/Victoria viruses are the most commonly reported influenza viruses among children age 0-4 years (46%) and 5-24 years (57%).
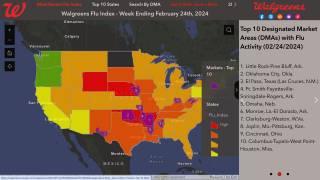
From a state focus, Influenza-Associated Pediatric Deaths by Health and Human Services (HHS) Region, it appears Region #6 is the unfortunate leader, with the state of Texas confirming 8 pediatric fatalities, as of December 19, 2019.
Influenza is an acute respiratory illness caused by influenza A or B viruses, and rarely influenza C viruses.
There are 2 types of Influenza B Viruses:
- B/Victoria: 50 B/Victoria lineage viruses, including viruses from both co-circulating sub-clades, were antigenically characterized by HI with ferret antisera, and 29 (58%) were antigenically similar to cell-propagated B/Colorado/06/2017-like reference viruses representing the B/Victoria component for the 2019-20 Northern Hemisphere influenza vaccines.
- B/Yamagata: 10 B/Yamagata lineage viruses were antigenically characterized by HI with ferret antisera, and all 10 (100%) were antigenically similar to cell-propagated B/Phuket/3073/2013-like reference viruses representing the B/Yamagata component for the 2019-20 Northern Hemisphere influenza vaccines.
Children commonly need medical care because of the flu, especially children younger than 5 years old. The flu’s signs and symptoms of upper and/or lower respiratory tract involvement are common, but the presentation varies with age and previous experience with influenza viruses.
The seasonal flu vaccine protects against the influenza viruses that research indicates will be most common during the upcoming season. Most flu vaccines protect against 4 different influenza viruses for the 2019-2020 Northern Hemisphere season, including influenza B.
The CDC recommends annual influenza vaccination for everyone 6 months and older with any licensed, influenza vaccine that is appropriate for the recipient’s age and health status, with no preference expressed for any one vaccine over another.
- Injectable influenza vaccines (IIV) are given as an injection and are approved for use in people 6 months and older.
- Live inactivated influenza vaccine is given as a nasal spray and is approved for use in non-pregnant people 2 through 49 years old. However, there is a precaution against the use of nasal spray flu vaccine in people with certain underlying medical conditions.
The CDC also suggests pregnant women get a flu shot and not the nasal spray influenza vaccine for the current flu season.
The CDC says antiviral medications against flu viruses are an important adjunct to influenza vaccines. The CDC published these details in November 2019, which are as follows:
- Influenza antiviral prescription drugs can be used to treat influenza, and some can be used to prevent influenza.
- There are 4 influenza antiviral medications approved by the U.S. Food and Drug Administration (FDA) recommended for use in the USA during the 2019-2020 influenza season.
- Three drugs are chemically related antiviral medications known as neuraminidase inhibitors that block the viral neuraminidase enzyme and have activity against both influenza A and B viruses: oral oseltamivir phosphate (Tamiflu), inhaled zanamivir (Relenza), and intravenous peramivir (Rapivab).
- The 4th drug is oral baloxavir marboxil (Xofluza), which is active against both influenza A and B viruses but has a different mechanism of action than neuraminidase inhibitors. Baloxavir is a cap-dependent endonuclease inhibitor that interferes with viral RNA transcription and blocks virus replication.
As always, vaccination decisions should be part of an ongoing discussion between a healthcare provider and patient.
Flu news published by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee