$34.8 Million Funds MERS Vaccine Candidate Through Phase 2

Barinthus Biotherapeutics plc today announced a project with the Coalition for Epidemic Preparedness Innovations (CEPI) and the University of Oxford, aiming to fast-track the development of the VTP-500 vaccine candidate for the prevention of Middle East Respiratory Syndrome (MERS) Coronavirus (CoV).
This new project intends to take this MERS vaccine through Phase II clinical trials and, if successful, on into the development of an investigational ready reserve of 100,000 doses.
The three-way partnership has awarded up to $34.8 million to Barinthus Bio in addition to funds previously committed to the University of Oxford.
VTP-500 has already completed Phase I clinical trials in Britain and Saudi Arabia, and the University of Oxford is now conducting a Phase Ib trial in the UK to assess vaccination of older adults, the age group most in need of this vaccine.
The VTP-500 development program was awarded PRIME designation in December 2023 by the European Medicines Agency.
This program is essential, as no vaccines targeting MERS have been licensed.
“We are thrilled to be working with the University of Oxford and CEPI on developing this important vaccine candidate,” commented Bill Enright, Barinthus Bio’s Chief Executive Officer, in a December 21, 2023 press release.
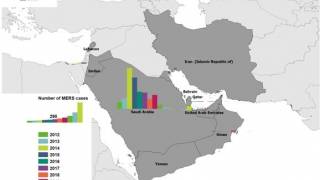
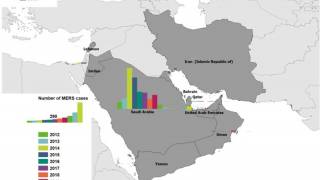
MERS is a severe respiratory infection caused by MERS-CoV, a coronavirus first identified in 2012 in Saudi Arabia. It has caused more than 2,600 human infections in at least 27 countries. MERS has a case-fatality rate of more than 35%.
The World Health Organization wrote on August 29, 2023, that it expects additional MERS-CoV cases to be reported from the Middle East and/or other countries where MERS-CoV is circulating in dromedaries.
Barinthus Bio, formerly Vaccitech plc, is a clinical-stage biopharmaceutical company developing novel T-cell immunotherapeutic candidates designed to guide the immune system to overcome chronic infectious diseases, autoimmunity, and cancer.
Our Trust Standards: Medical Advisory Committee