$3 Million Grant Supports Bi-Specific Malaria Vaccine Development

Versatope Therapeutics Incorporated announced today it has received a Phase 2 Small Business Innovation Research (SBIR) grant for up to $3 million over three years from the U.S. NIH's National Institute of Allergy and Infectious Diseases.
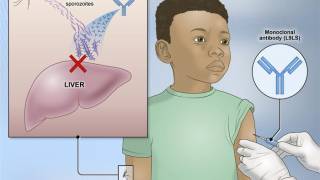
On July 29, 2024, Versatope confirmed it will use the grant (#R44AI181242) to develop a bi-specific malaria vaccine using a target that blocks the initial malaria infection and transmission.
The Company says the novel, dual-acting vaccine may offer a more robust approach than the current World Health Organization (WHO) certified single-acting malaria vaccines.
Versatope was also awarded a Stage I grant from the MassVentures SBIR Targeted Technologies program.
"We appreciate the recognition and support of the NIH and MassVentures team to advance the development of Versatope's technology platform and to help take the company to the next stage of development," said Christopher Locher, CEO of Versatope, in a press release.
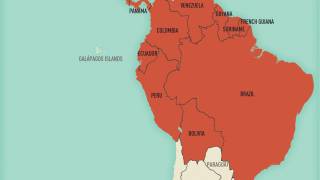
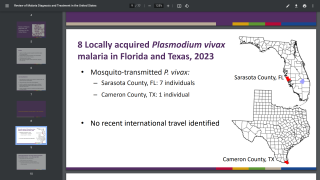
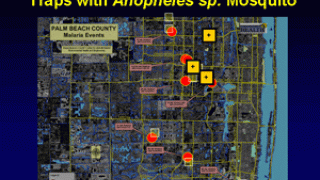
As of July 2024, two malaria vaccines are being deployed in various countries.
For example, the African country of Côte d'Ivoire recently became the first nation to deploy the R21/Matrix-M™ vaccine.
"The introduction of the R21/Matrix-M™ malaria vaccine in Côte d'Ivoire marks a breakthrough in the fight to protect vulnerable children against a leading cause of death across the region while reinforcing our mission to create innovative vaccines that improve public health," said John Jacobs, Novavax Inc.'s President and CEO, said in a press release on July 15, 2024.
Our Trust Standards: Medical Advisory Committee