Pneumonia Remains the Forgotten Vaccine-Preventible Disease
The U.S. Centers for Disease Control and Prevention (CDC) reported today the number of pneumonia-related fatalities continues to outpace both COVID-19 and Influenza.
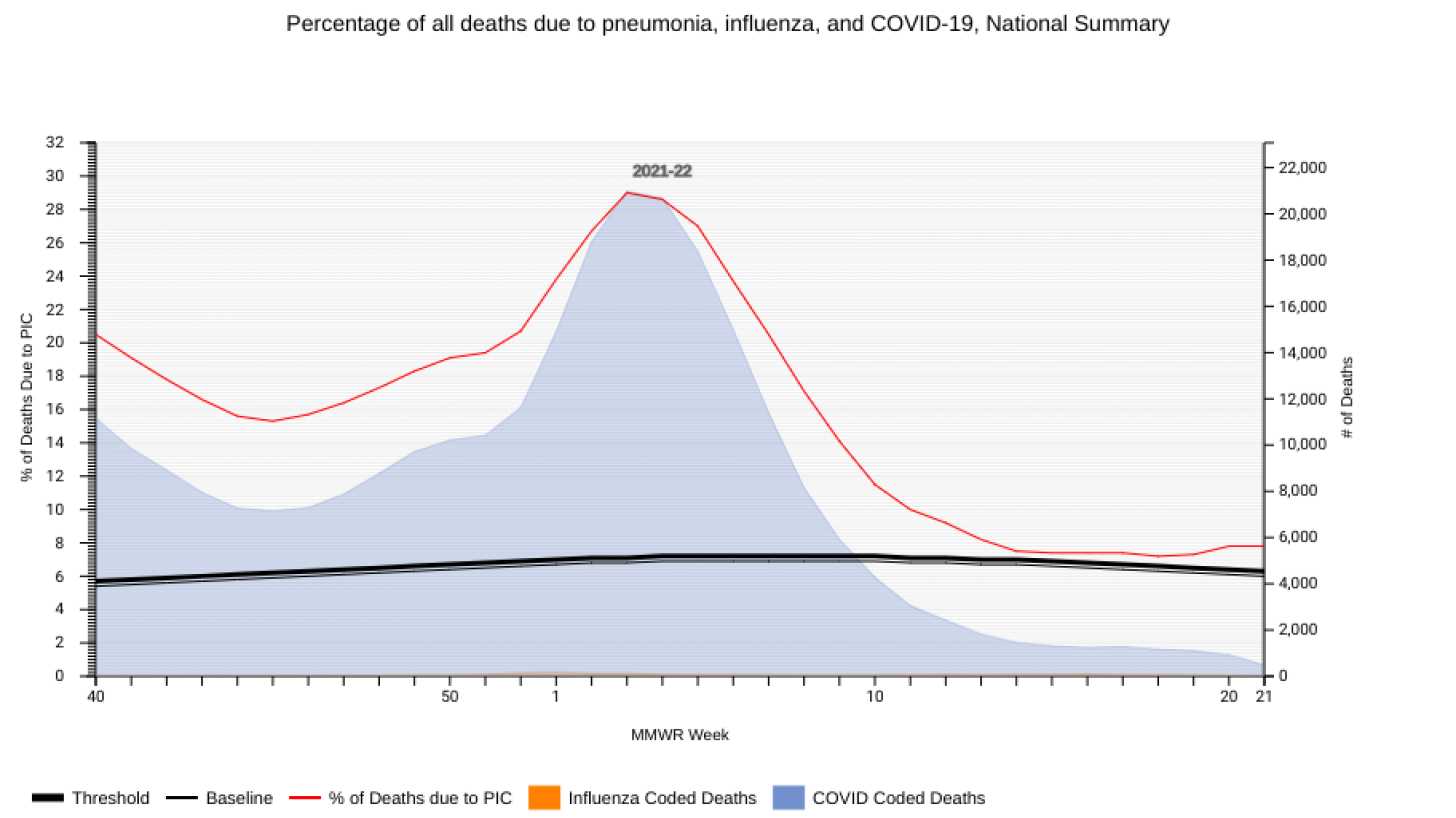
The CDC's National Center for Health Statistics (NCHS) Mortality Surveillance includes data evaluating pneumonia, influenza, and COVID-19 (PIC) fatalities.
As of June 17, 2022, data collected (82%) for week #19 indicates there were 2,074 pneumonia fatalities in the USA, far surpassing COVID-19 (1,103) and influenza (57).
And the CDC reported the current 7-day moving average of new COVID-19-related fatalities (306) had increased 18.6% compared with last week. So as of June 8, 2022, a total of 1,005,823 COVID-19 deaths have been reported in the United States.
Historically, pneumonia and influenza cases produced significantly fewer fatalities. For example, in 2020, pneumonia-related deaths were 47,601, while influenza deaths were 5,943.
Pneumonia is an infection of the lungs that can cause mild to severe illness in people of all ages. Viruses, bacteria, and fungi can all cause pneumonia. And these bacteria can cause a wide range of infections—like pneumonia—known as pneumococcal disease.
In the USA, common causes of viral pneumonia are influenza, respiratory syncytial virus, and SARS-CoV-2 viruses. A common cause of bacterial pneumonia is Streptococcus pneumoniae.
The good news is pneumonia is a vaccine-preventable disease, and the U.S. FDA has approved two types of pneumococcal vaccines, PCV13, and PPSV23.
However, neither vaccine can prevent every kind of community-acquired pneumonia, but they work against the 30 most common bacteria types.
And other FDA-approved vaccines can help prevent pneumonia available as of June 17, 2022, including:
- COVID-19
- Haemophilus influenzae type b (Hib)
- Influenza
- Measles
- Pertussis
- Varicella
Unfortunately, the CDC's data shows that in 2020, the percentage of adults who received a pneumococcal vaccination was just 25.5%.
In an editorial written by AMIT A. SHAH, MD, AGSF, Mayo Clinic College of Medicine and Science, and published by the American Academy of Family Physicians, current real-world data was highlighted, which is excerpted below:
"With the 2021 approval of PCV15 (Vaxneuvance) and PCV20 (Prevnar 20), the ACIP has greatly simplified pneumococcal vaccination recommendations."
PCV15 and PCV20 are conjugate vaccines that provide durable protection because of a T cell-dependent mechanism of action and resulting memory B cell formation that provide mucosal immunity.
In contrast, PPSV23, a capsular polysaccharide vaccine that induces an immune response via the release of immunoglobulin from B cells, does not result in mucosal immunity, and protection wanes over five to six years.
Compared with the PCV13 vaccine, the PCV15 and PCV20 vaccines cover additional strains that cause about 15% and 27% of invasive pneumococcal disease in patients 65 years or older and 13% and 28% of disease in patients 19 to 64 years of age with underlying conditions, respectively.
'Studies have shown that the overall immunogenicity of PCV20 alone or PCV15 plus PPSV23 is similar to PCV13 plus PPSV23. Therefore, PCV15 and PCV20 are expected to be safe and well-tolerated, with no serious adverse events noted in clinical trials,' wrote Dr. Shah.
The CDC published an updated vaccination schedule in February 2022, highlighting recommendations from CDC's Advisory Committee on Immunization Practices.
PrecisionVaccinations publishes fact-checked, research-based vaccine news curated for mobile readership.
Our Trust Standards: Medical Advisory Committee
























