21 Serotype Pneumococcal Vaccine Approved for Adults

Merck announced today that the U.S. Food and Drug Administration (FDA) has approved CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine).
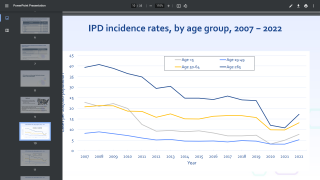
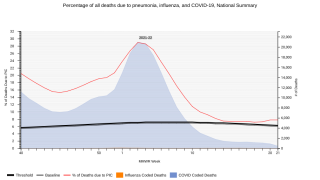
CAPVAXIVE (V116) is specifically designed to help protect adults against the serotypes that cause most invasive pneumococcal disease (IPD) cases. CAPVAXIVE includes eight unique serotypes not covered by other currently approved pneumococcal vaccines.
This innovative vaccine is for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15B, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F and 35B in individuals 18 years of age and older.
And active immunization for the prevention of pneumonia caused by S. pneumoniae serotypes 3, 6A, 7F, 8, 9N, 10A, 11A, 12F, 15A, 15C, 16F, 17F, 19A, 20A, 22F, 23A, 23B, 24F, 31, 33F and 35B in individuals 18 years of age and older.
The U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) is expected to meet in June 2024 to discuss and make recommendations for the use of CAPVAXIVE in adults.
The ACIP met on February 29, 2024, when Heather Platt, M.D., presented Key Results from the Phase 3 Clinical Development Program. Based on CDC data, the serotypes covered by CAPVAXIVE are responsible for more cases of IPD in adults than PCV20 (pneumococcal 20-valent conjugate vaccine).
“Today’s approval is a testament to our population-specific strategy behind CAPVAXIVE, which demonstrated robust immunogenicity in a range of adult populations and is driven by a deep understanding of pneumococcal disease,” said Dr. Dean Y. Li, president of Merck Research Laboratories, in a press release on June 17, 2024.
“We are proud to provide CAPVAXIVE as a new option specifically designed to help protect against the majority of invasive pneumococcal disease-causing serotypes in adults.”
CAPVAXIVE is administered as a single dose, and is the sixth FDA-approved product to utilize the Pfenex Expression Technology® platform.
Current ACIP vaccine recommendations are posted at this CDC link.
Our Trust Standards: Medical Advisory Committee