15-Valent Pneumococcal Conjugate Vaccine Reports Positive Phase 3 Results

Two additional Phase 3 studies evaluating the safety, tolerability, and immunogenicity of V114, a Merck investigational 15-valent pneumococcal conjugate vaccine were announced.
Merck’s press release on October 20, 2020, stated ‘V114 was generally well-tolerated in both studies, with a safety profile consistent with that observed for V114 in previously reported studies.’
V114 is Merck’s investigational 15-valent pneumococcal conjugate vaccine in development for the prevention of pneumococcal disease in adults and children. V114 consists of pneumococcal polysaccharides from 15 serotypes conjugated to a CRM197 carrier protein and includes serotypes 22F and 33F, which are commonly associated with the invasive pneumococcal disease worldwide and are not contained in the pneumococcal conjugate vaccine currently licensed for use in adults.
Dr. Roy Baynes, senior VP and head of global clinical development, CMO, Merck Research Laboratories, stated: “These data provide important information about the potential for V114 followed by PNEUMOVAX 23, a polysaccharide vaccine included in more than 90 percent of age-based adult pneumococcal immunization programs globally, to help protect healthy adults and adults who are at increased risk for pneumococcal disease.”
In the PNEU-PATH (V114-016) study, healthy adults 50 years of age or older received V114 or PCV13, followed by PNEUMOVAX® 23, one year later. This study found the immune responses following vaccination with PNEUMOVAX 23 (month 13) were comparable in both vaccination groups for the 15 serotypes in V114.
Results also showed that at 30 days post-vaccination with either V114 or PCV13, immune responses were comparable for both groups across the 13 serotypes shared by the conjugate vaccines and higher in the V114 group for serotypes 22F and 33F, the two serotypes not included in PCV13.
In the PNEU-DAY (V114-017) Phase 3 study, immunocompetent adults 18 to 49 years of age with underlying medical conditions associated with increased risk for pneumococcal disease, V114 generated immune responses generally comparable to PCV13 for the 13 shared serotypes and higher immune responses for serotypes 22F and 33F at 30 days post-vaccination.
These findings from the V114 Phase 3 clinical program in adults, including PNEU-PATH and PNEU-DAY, will be presented at a future scientific congress. Plans for global regulatory licensure applications, beginning with the U.S. Food and Drug Administration (FDA) before the end of 2020, remain on track.
Results from both studies are based on opsonophagocytic activity (OPA) responses – a measure of vaccine-induced functional antibodies.
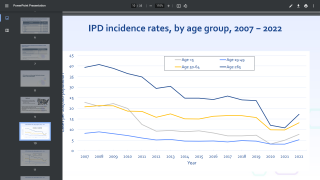
Pneumococcal serotypes not in the currently licensed conjugate vaccine, such as 22F and 33F, are among the most common serotypes causing invasive pneumococcal disease in parts of the world, including the U.S., among adults 65 years of age or older.
Invasive pneumococcal disease due to serotypes 22F and 33F has been linked to higher case fatality rates and prolonged hospitalization in adults. Overall, adults with certain medical conditions, such as heart disease, diabetes, or chronic obstructive pulmonary disease (COPD), have a higher risk for pneumococcal disease compared to those without these conditions.
The V114 Phase 3 clinical development program is comprised of 16 trials investigating the safety, tolerability, and immunogenicity of V114 in a variety of populations who are at increased risk for pneumococcal disease, including healthy older adults and children, as well as people who are immunocompromised or have certain chronic medical conditions.
About Pneumococcal Disease
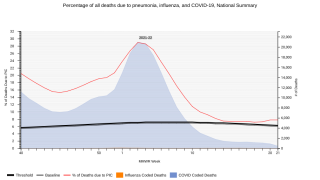
The global prevalence of pneumococcal disease, an infection caused by bacteria called Streptococcus pneumoniae, is evolving. There are more than 90 different types of pneumococcal bacteria which can affect adults differently than children.
Highly aggressive strains, or serotypes, threaten to put more people at risk for non-invasive pneumococcal illnesses such as pneumococcal pneumonia, sinusitis, and otitis media, and invasive pneumococcal illnesses such as pneumococcal bacteremia (infection in the bloodstream), bacteremic pneumonia (pneumonia with bacteremia) and pneumococcal meningitis (infection of the coverings of the brain and spinal cord).
While healthy adults and children can suffer from pneumococcal disease, patient populations particularly vulnerable to infection include children under the age of 2, older adults such as those 65 years of age and older, and people with immunosuppressive or certain chronic health conditions.
An updated overview of pneumococcal vaccines is listed on this PrecisionVaccinations webpage.
PrecisionVaccinations publishes research-based vaccine development news.
Our Trust Standards: Medical Advisory Committee