DNA Vaccine Candidate Launches Phase 2 Study Against MERS

Pennsylvania-based INOVIO announced today that the company had dosed the first Phase 2 trial subject in its quest to develop the first vaccine against the Middle East Respiratory Syndrome (MERS).
INOVIO designed its DNA vaccine candidate INO-4700 to prevent MERS, a disease in the coronavirus family for which there are no U.S. FDA-Approved vaccines.
The multi-center Phase 2 trial is a randomized, double-blinded, placebo-controlled study designed to evaluate the safety, tolerability, and immunogenicity of INO-4700 administered with INOVIO's smart device, the CELLECTRA® 2000, in approximately 500 healthy adult volunteers.
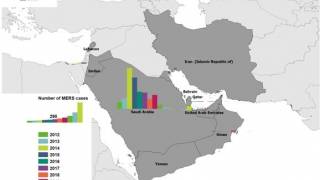
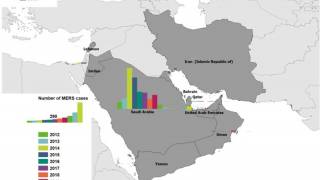
The study is sponsored by INOVIO and fully funded by the Coalition for Epidemic Preparedness Innovations (CEPI), is being conducted at sites in Jordan and Lebanon where MERS cases have been reported.
This trial builds on the positive results of the Phase 1 trial, published in a peer-reviewed article in The Lancet Infectious Diseases. Study results found high levels of binding antibodies in 92% of evaluated subjects. In addition, significant antigen-specific cytotoxic T-lymphocyte (CTL) responses were also observed.
Importantly, 98% of vaccinated subjects generated an antibody and/or T cell response against the MERS vaccine.
Dr. J. Joseph Kim, President and CEO of INOVIO, said in a press statement, "This advancement not only complements our late-stage efforts with COVID-19, but it also represents an important milestone for INOVIO's infectious disease platform."
"We look forward to continuing our collaboration with CEPI and moving another step closer to providing patients with a safe and effective preventive vaccine against MERS."
INOVIO and CEPI stated they plan to make a stockpile of these vaccines available for emergency use as soon as possible following Phase 2 testing.
Since the MERS virus was first identified in Saudi Arabia in 2012, more than 2,580 cases of MERS-CoV have been detected in 27 countries, reported the ECDC in July 2021.
MERS causes a rapidly progressive respiratory illness that may require intensive care treatment and mechanical ventilation in many patients, says the U.S. CDC. The source of the virus remains unknown, but the pattern of transmission and virological studies point toward dromedary camels in the Middle East as a reservoir from which humans sporadically become infected through zoonotic transmission.
In addition, human-to-human transmission of MERS is amplified among household contacts and in healthcare settings.
INOVIO's pursuit of a MERS vaccine is funded by a previously announced $56 million grant from CEPI. INOVIO is advancing two vaccine candidates through Phase 2 field trials against MERS and Lassa fever, respectively.
INOVIO has 15 DNA medicine clinical programs currently in development, including coronaviruses associated with MERS and COVID-19 diseases being developed under grants from the Coalition for Epidemic Preparedness Innovations and the U.S. Department of Defense.
Note: DNA medicines are composed of optimized DNA plasmids, which are small circles of double-stranded DNA synthesized or reorganized by a computer sequencing technology and designed to produce a specific immune response in the body.
Our Trust Standards: Medical Advisory Committee