Pneumococcal 15-valent Conjugate Vaccine for Infants and Adolescents Approval Delayed

New Jersey-based Merck recently announced that the U.S. Food and Drug Administration (FDA) had extended the Prescription Drug User Fee Act date of the supplemental biologics license application for VAXNEUVANCE™ (Pneumococcal 15-valent Conjugate Vaccine) for infants and adolescents to July 1, 2022.
The initial target action date for children six weeks through 17 years of age was April 1, 2022.
The FDA requested additional data analyses from the pediatric studies, which Merck has submitted to the FDA.
And no new clinical studies have been requested by the FDA.
In December 2021, Merck announced that the FDA accepted the company’s application for VAXNEUVANCE to prevent invasive pneumococcal disease in children and adolescents and was granted Priority Review.
“We are confident in the strength of the data from our pediatric studies with VAXNEUVANCE and will continue to work expeditiously with the FDA to bring this important vaccine forward to infants and children in the United States as soon as possible,” said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories, in a press release issued on April 1, 2022.
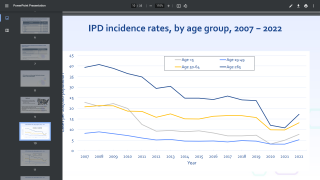
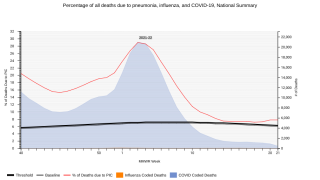
This pending approval is vital since children under the age of 2 are particularly vulnerable to pneumococcal infection, and the incidence of invasive pneumococcal disease remains highest in the first year of life, says the U.S. CDC.
However, vaccination with VAXNEUVANCE may not protect all vaccine recipients, says Merck.
The FDA approved VAXNEUVANCE for adults on July 16, 2021, and Europe issued its approval in December 2021.
Additional pneumococcal vaccine news is posted on this weblink.
Note: The Merck statement was edited for clarity and manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee