Pneumococcal 15-valent Conjugate Vaccine Indication Expanded

New Jersey-based Merck announced today that the U.S. Food and Drug Administration (FDA) had approved an expanded indication for its VAXNEUVANCE™ Pneumococcal 15-valent Conjugate Vaccine to include children six weeks through 17 years of age.
VAXNEUVANCE is now indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F in individuals six weeks of age and older.
Today's approval follows the FDA's Priority Review of Merck's supplemental application.
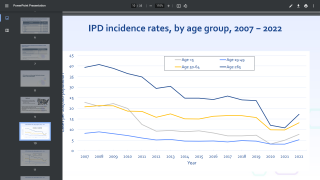
"Despite decreases in incidence of invasive pneumococcal disease (IPD) in children, certain key serotypes continue to cause serious illness that can lead to death in children under the age of 5, with serotypes 3, 22F, and 33F responsible for more than 25% of all IPD cases in this population," commented Dr. Steven Shapiro, chairman, department of pediatrics, Jefferson Abington Hospital, and investigator for the PNEU-PED trial, in a related press release issued on June 22, 2022.
"With the robust clinical data supporting VAXNEUVANCE and this FDA approval, VAXNEUVANCE will be an important new option to help advance protection for children."
IPD is an infection caused by the bacterium Streptococcus pneumoniae or pneumococcus. While there are approximately 100 different types of S. pneumoniae, called serotypes, a smaller number of serotypes are responsible for IPD in children.
Children under the age of 2 are particularly vulnerable to IPD.
The FDA initially approved VAXNEUVANCE in July 2021.
The FDA previously granted VAXNEUVANCE Breakthrough Therapy designation and Priority Review for the pediatric indication.
Note: This Merck media statement was manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee