Pneumococcal 21-valent Conjugate Vaccine Recommended for Appropriate Adults

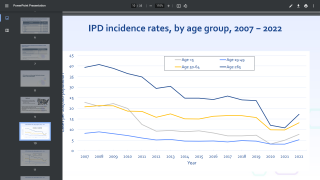
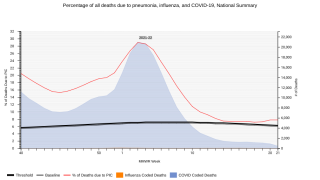
Merck announced today that the U.S. CDC’s Advisory Committee on Immunization Practices (ACIP) unanimously voted to recommend CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine) as an option for adults 65 years of age and older for pneumococcal vaccination.
Additionally, shared clinical decision-making is recommended regarding using a supplemental dose of CAPVAXIVE for adults 65 and older who have completed their vaccine series with both PCV13 and PPSV23.
“CAPVAXIVE represents an innovative approach to invasive pneumococcal disease prevention in adults, as it is specifically designed to help protect against the strains that cause the majority of severe disease in adults 65 years of age and older,” said Dr. Eliav Barr, senior vice president, Merck Research Laboratories, in a press release on June 27, 2024.
“The ACIP vote recognizes the clinical profile of CAPVAXIVE for adults in the U.S., and we look forward to the CDC’s final, published recommendations.”
Our Trust Standards: Medical Advisory Committee