Zaire Ebola Vaccine Found 84% Effective

According to real-world evidence published in The Lancet Infectious Diseases today, this analysis is the first to provide estimates of Merck's Ervebo® (rVSV-ZEBOV) vaccine against Zaire Ebolavirus disease amid the widespread use of the vaccine during a large outbreak.
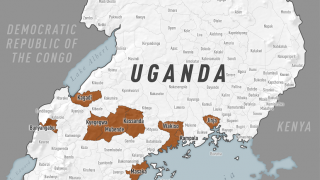
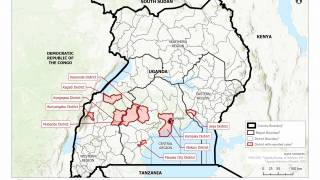
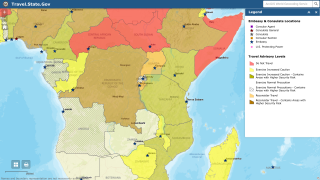
Announced on August 20, 2024, these findings confirm that Ervebo is highly protective against 84% (95% credible interval, 70% to 92%) of Ebolavirus disease and supports its use during outbreaks, even in challenging contexts such as in the eastern Democratic Republic of the Congo (DRC).
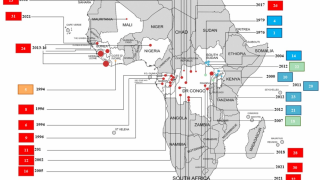
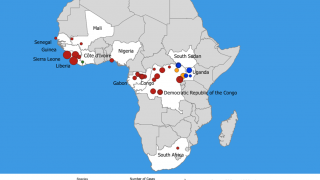
This finding is essential since Ebolaviruses are endemic in the DRC.
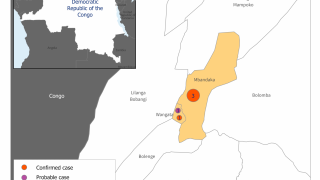
In a related Editorial, the authors wrote the 2018–20 Ebola virus disease epidemic in the DRC resulted in 3,470 reported cases and remains the second-largest Ebolavirus outbreak in recorded history worldwide. The initial Ebola outbreak was in 1976.
In November 2019, the World Health Organization prequalified the Ervebo vaccine. The U.S. Food and Drug Administration approved it on December 19, 2019.
Médecins Sans Frontières (Doctors Without Borders) funded this study.
Our Trust Standards: Medical Advisory Committee