Pneumococcal Disease Vaccine Candidate Launches Phase 1 Study

The first subject has been dosed with ASP3772, a novel Multiple Antigen Presenting System (MAPS) vaccine candidate targeting Streptococcus pneumoniae (pneumococcus), announced Affinivax, Inc.
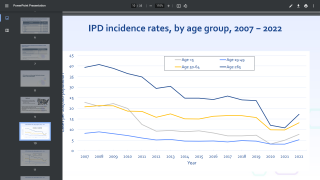
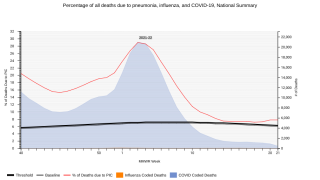
This is important news, since Streptococcus pneumoniae is the leading cause of lower respiratory infection morbidity and mortality globally, contributing to more deaths than all other etiologies combined in 2016.
In the USA, there are over 900,000 cases of pneumococcal pneumonia each year.
This limited, clinical study protocol is “A Phase 1/2, Randomized, Single Ascending Dose Study in Adults (Stage 1) and Randomized, Single Ascending Dose-Finding Study in Elderly Subjects (Stage 2) with ASP3772.
The MAPS technology platform uses proprietary chemistry that capitalizes on the highly specific and durable non-covalent, affinity binding between biotin and rhizavidin, a biotin-binding protein.
The highly stable complex created by this affinity binding contributes to a simple, modular, and efficient approach to the development of MAPS vaccines.
In stark contrast to the highly complex chemistry of conventional vaccine conjugation (which is optimized to induce protective antibody responses to only the polysaccharide antigen with the protein antigen serving primarily as a carrier), a MAPS vaccine can present both the polysaccharide antigen and the protein antigen to induce a broad and potent immune response, said Affinivax in a press release.
Richard Malley, MD, Affinivax’s Scientific Founder, Professor of Pediatrics at Harvard Medical School, and the Kenneth McIntosh Chair in Pediatric Infectious Diseases at Boston Children’s Hospital, said in a press release, “We believe that MAPS, in contrast to traditional vaccines, offers a unique way to present the key epitopes of the desired antigens (polysaccharides and/or proteins) to induce a robust and broad immune response. We now look forward to confirming this in clinical trials."
Streptococcus pneumoniae (pneumococcus) is a bacterium frequently found in the upper respiratory tract of healthy children and adults, and can cause serious infections ranging from pneumonia, meningitis, and sepsis, says the Centers for Disease Control and Prevention.
If approved by the Food and Drug Administration, ASP3772 would compete with the Prevnar 13® vaccine.
For more information, visit Affinivax.
Our Trust Standards: Medical Advisory Committee
- Affinivax, Inc. announces initiation of Phase 1/2 clinical study of ASP3772, its novel pneumococcal MAPS vaccine
- A Single Ascending Dose Study in Adults (Stage 1) and Single Ascending Dose-Finding Study (Stage 2) in Elderly Subjects
- Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195
- Pneumococcal Disease (Streptococcus pneumoniae)