Phase 3 Studies on PCV-15 Launched

While most people have heard of pneumonia, many are confused who pneumococcal disease impacts.
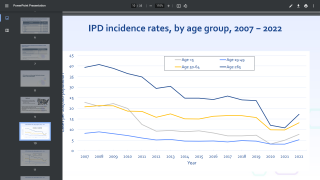
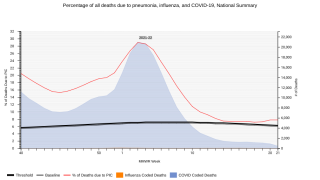
Each year in the United States, pneumococcal disease kills thousands of adults, including 16,000 adults aged 65+ years.
Pneumococcal disease is an infection caused by Streptococcus Pneumoniae bacteria, sometimes referred to as pneumococcus.
Pneumococcus can cause many types of illnesses, including pneumonia, ear infections and meningitis, reports the Centers for Disease Control and Prevention (CDC).
The best way to prevent pneumococcal disease is to get an approved vaccine(s).
There are 2 types of approved pneumococcal vaccines:
- The PCV13 vaccine for infants, older adults, and people with certain health conditions,
- The PPSV23 vaccine for children and adults age 2 and older, older adults, people with certain health conditions, and adults ages 19 through 64 who smoke.
And, Merck, the manufacturer of these vaccines, is beginning two Phase 3 studies of PCV-15 (V114), its investigational polyvalent conjugate vaccine for the prevention of pneumococcal disease.
**** Find a clinical trial in 60 seconds ****
The decision to move PCV-15 to Phase 3 studies is based on the findings of previous Phase 1 and Phase 2 studies, the results of which are being presented at the upcoming International Society on Pneumococci and Pneumococcal Diseases conference.
The first Phase 3 study (NCT03480763) will evaluate the safety, tolerability, and immunogenicity of PCV-15 followed by Pneumococcal Vaccine Polyvalent 1 year later in healthy adult subjects 50 years of age or older.
The second Phase 3 study (NCT03480802) is evaluating the safety, tolerability and immunogenicity of PCV-15 followed by Pneumococcal Vaccine Polyvalent administered 8 weeks later in adults infected with human immunodeficiency virus (HIV).
These advanced studies are focused on expanding the use of the PCV-15 vaccine, which is needed research.
But, previous research found getting pneumococcal vaccines prescribed is also an issue.
A study in 2017 indicated that primary care providers and physician assistants often fail to encourage vaccination against pneumococcus in patients who had asthma or were active smokers.
But, pharmacists embedded in family practice clinics were most likely to vaccinate patients. The pharmacists who participated in this study were able to spend more time with patients as they manage chronic conditions.
The CDC recommends these vaccines for all adults 65 years or older.
It is also recommended for children and adults 2 through 64 years old who are at increased risk for pneumococcal disease.
Because there are more than 90 known pneumococcal serotypes that cause disease, a previous pneumococcal infection will not protect you from a future infection.
Therefore, the CDC recommends pneumococcal vaccines for both children and adults who have had the pneumococcal disease in the past.
Most government and private health insurance policies cover pneumococcal vaccines.
The CDC Vaccine Price List provides private sector vaccine prices for general information. Vaccine discount information in the USA can be found here.
Vaccines, like any medicine, can have side effects, says the CDC. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
Our Trust Standards: Medical Advisory Committee
- A Study of Pneumococcal Conjugate Vaccine (V114) Compared to a Marketed Vaccine (V114-003 AM2)
- Merck Announces First Phase Three Studies for PCV-15 (V114) Its Investigational Pneumococcal Disease Vaccine
- Immunogenicity differences of a 15-valent pneumococcal polysaccharide conjugate vaccine (PCV15) based on vaccine dose, route of
- The Pneumocell-study: Vaccination of IgG1- and IgG2-deficient patients with Prevnar13
- Evaluation of a 15-valent Pneumococcal Conjugate Vaccine in an Adult Rhesus Macaque Immunogenicity Model