40th Country Authorizes Second-Gen Dengue Vaccine

At the end of July 2024, Swissmedic authorized Takeda Pharma AG's QDENGA® (TAK-003) Tetravalent Vaccine (Live, Attenuated) vaccine after assessing its efficacy, safety, and quality.
According to Swissmedic's statement on August 2, 2024, QDENGA is authorized for people aged four years and older who travel to regions where dengue fever is prevalent. These areas primarily include subtropical and tropical regions in Central Africa, Latin America, India, and Southeast Asia.
Swissmedic says QDENGA cannot cause the disease, but vaccination triggers the immune system to defend the body against the virus. When a person receives the vaccine, their immune system recognizes the attenuated variants as foreign and forms antibodies against them. When they come into contact with the virus again, the body rapidly produces more antibodies to neutralize it before the person contracts dengue fever.
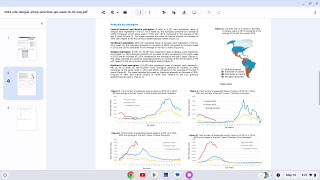
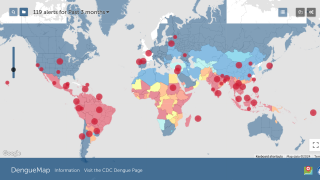
The U.S. Centers for Disease Control and Prevention (CDC) recently announced that the global incidence of dengue this year is the highest in recent history, with about 100 countries reporting higher-than-usual dengue cases.
In 2024, both travel-related and locally transmitted dengue infections were confirmed in the south of the USA, in states such as Florida (335) and Texas (18).
Dengue fever is a viral disease spread by infected mosquitoes. It usually develops between four and seven days after the person is bitten. Symptoms include fever, headache, pain behind the eyes, muscle and joint pain, nausea or vomiting, swollen glands, and rash.
As of August 21, 2024, QDENGA had been approved in 40 countries and launched (or was available) in 24 of them.
Note: This new article was updated with information on current vaccine availability with a changed headline on August 23, 204.
Our Trust Standards: Medical Advisory Committee