Canada Prepares for Rare Diseases

Takeda Canada Inc. today annonced a new report, Enhancing Diagnosis, Access, Care, and Treatment, highlighting the urgent need for innovative funding models and collaboration to help accelerate Canada’s National Strategy for Drugs for Rare Diseases.
Nearly 200 novel drugs for rare diseases are being developed and are expected to launch in Canada within the next ten years. It’s estimated only 5% of rare diseases have an approved treatment.
A “rare” disease is any disease that affects a minimal number of individuals. It is often genetic, chronic throughout a patient’s life, and life-threatening. With rare diseases affecting relatively limited patients, innovative treatments are often unavailable.
The impact of rare diseases is significant, with approximately one in 12 Canadians, two-thirds of whom are children.
“Canadians living with rare diseases have every reason to be optimistic,” says Durhane Wong-Rieger, President & CEO of the Canadian Organization for Rare Disorders, in a press release on August 22, 2024.
“Hundreds of new therapies are being developed, many targeting the 95% of rare diseases with no known treatment! We must leverage the $1.5 billion Rare Disease Drug Strategy,
The journey toward appropriately managing a rare disease is long and challenging. On average, it takes 6 to 8 years before a patient receives a correct diagnosis; this time, they will see an average of eight physicians and receive two to three misdiagnoses.
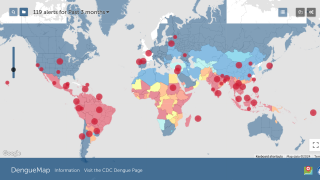
Takeda also produces innovative products, such as QDENGA®, an approved two-dose vaccine that prevents dengue fever and/or severe dengue in adults caused by any of the four serotypes of the dengue virus.
This dengue vaccine is authorized in about 40 countries and does not require pre-admission testing.
Our Trust Standards: Medical Advisory Committee