Dengue Antiviral Development Discontinued

Millions of people will be infected with the Dengue virus in the last few months of 2024 without being able to be treated with an approved antiviral.
Johnson & Johnson (J&J) announced on October 4, 2024, that it discontinued the Phase 2 field study evaluating the efficacy of investigational antiviral candidate mosnodenvir (JNJ-1802) for the prevention of dengue virus in adults.
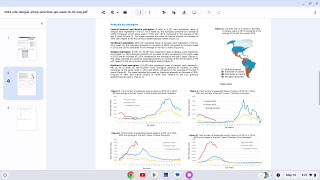
Recent results from the Phase 2a human challenge study found that the compound induced antiviral activity against one of dengue's four viruses (DENV-3) in humans, compared to placebo.
J&J's decision to discontinue this study is part of a strategic reprioritization of the Company’s Communicable Diseases research and development portfolio. However, J&J will continue to support the fight against dengue by sharing study results with the medical community in the future.
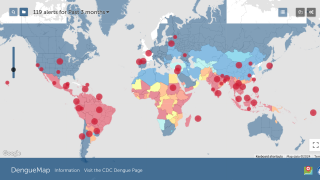
Almost 4 billion people will live in areas at risk of dengue in 2024, including 26 countries recently highlighted by the U.S. Centers for Disease Control and Prevention (CDC).
According to the U.S. CDC, dengue symptoms can become severe within a few hours. See a healthcare provider if you develop a fever or have symptoms of dengue. Dengue can be found in the blood during the first week of illness.
Furthermore, time is of the essence as severe dengue is a medical emergency.
Dengue is a vaccine-preventable disease. Various approved vaccines have limited availability, and candidate vaccines are conducting late-stage research in October 2024.
Our Trust Standards: Medical Advisory Committee